Статтю опубліковано на с. 14-24
Вступ
Цукровий діабет (ЦД) все ще залишається найбільш значущою екстрагенітальною патологією в акушерській практиці та характеризується 2–4-кратним збільшенням ризику багатьох ускладнень (гестаційна гіпертензія, прееклампсія, передчасні пологи), значно вищими показниками перинатальної смертності порівняно із загальною популяцією вагітних жінок [2]. Протягом багатьох років вагітність у жінок із діабетичною нефропатією розглядалась як ще більш ризикована, оскільки асоціювалась із можливістю прогресуючого зниження функціонального стану нирок, швидкого розвитку термінальних стадій ниркової недостатності та, як наслідок, високими показниками материнської смертності.
У загальній популяції вагітних жінок із ЦД 1-го типу діабетична нефропатія реєструється в 5–10 % пацієнток та належить до одного з найпоширеніших хронічних захворювань нирок під час вагітності [5].
Характеризуючи вплив вагітності на перебіг діабетичної нефропатії, слід визнати, що сьогодні існують лише поодинокі дослідження з вивчення віддаленого впливу вагітності на функціональний стан нирок у жінок із діабетичною нефропатією, але отримані в них результати є досить суперечливими. Окремими дослідженнями встановлено, що у випадках із нормальним початковим рівнем сироваткового креатиніну вагітність не асоціювалась із більш інтенсивним погіршенням ниркової функції, а також із зниженням показника виживання пацієнток [11]. В інших дослідженнях було продемонстровано, що в пацієнток із наявними порушеннями ниркової функції (III–V стадії хронічної хвороби нирок; рівень протеїнурії > 1 г/24 год; швидкість клубочкової фільтрації до вагітності ≤ 60 мл/хв; кліренс креатиніну < 90 мл/хв; рівень сироваткового креатиніну > 124 мкмоль/л) патологічні зміни поглиблювалися значно швидше під час самої вагітності та в післяпологовому періоді, ніж це можна було очікувати за відсутності вагітності в анамнезі, незалежно від адекватності глікемічного контролю та попереднього застосування нефропротекторних препаратів (зокрема, інгібіторів ангіотензинперетворюючого ферменту). До того ж значно раніше спостерігався розвиток термінальних стадій ниркової недостатності із виникненням потреби в нирковій замісній терапії [2, 3, 5, 9, 11].
Наявні в літературі дані свідчать про те, що діабетична нефропатія, зі свого боку, також може значно негативно впливати на перебіг вагітності та в результаті суттєво погіршувати перинатальні та материнські наслідки. Результати систематичних оглядів вказують на те, що за наявності діабетичної нефропатії ймовірність несприятливих наслідків для плода зростає вдвічі, а для матерів — у 5 разів [10]. Вважається, що діабетична нефропатія опосередковує свій негативний ефект через три основні механізми: розвиток тяжкої гіпертензії із подальшим порушенням та/або погіршенням функціонального стану нирок у матері; передчасне розродження у зв’язку з вираженим підвищенням рівня артеріального тиску (АТ) або розвитком прееклампсії. У цьому контексті було б доречно навести результати ретроспективного дослідження D. Carr та співавт. [2], які підтвердили, що в популяції пацієнток із діабетичною нефропатією субоптимальний контроль гіпертензії в ранні терміни вагітності (зокрема, рівень середнього АТ > 100 мм рт.ст.) асоціювався із значним ризиком передчасного розродження в терміни гестації < 32 тижнів. У нещодавно проведених дослідженнях було встановлено, що навіть у жінок із І стадією хронічної хвороби нирок (у тому числі діабетичного походження) виникнення або наявність артеріальної гіпертензії (АГ) збільшували ризик передчасних пологів в 3,40–7,24 раза [6, 7].
Третій основний механізм полягає в порушенні плацентації та, як наслідок, формуванні первинної плацентарної дисфункції із подальшим народженням «малих для гестацiйного віку» дітей (згідно з останнім, опублікованим у 2007 р. звітом британського Комітету конфіденційних розслідувань материнської та перинатальної смертності, в 31 % випадків вагітності з наявною до цього діабетичною нефропатією), формуванні синдрому затримки росту плода (в 64 % спостережень) або розвитком дистресу плода [2].
Загалом такі фактори визнаються клінічними предикторами сприятливого перебігу вагітності в пацієнток із наявною в анамнезі діабетичною нефропатією: незначне підвищення рівня сироваткового креатиніну (< 124 мкмоль/л); помірна протеїнурія (< 1 г на добу) та нормальний рівень АТ. Навпаки, високі концентрації креатиніну (> 176 мкмоль/л, а за деякими джерелами, > 124 мкмоль/л), протеїнурія в межах нефротичних значень (> 3 г на добу), рецидивуючі інфекції сечовидільних шляхів, наявність в анамнезі захворювань серцево-судинної системи та тяжка АГ асоціюються із несприятливими наслідками вагітності для матері та плода [2, 3, 5–7, 10, 11]. Усі вищенаведені дані свідчать про те, що за наявності діабетичної нефропатії саме гіпертензивні розлади (як існуючі до цього, так і індуковані вагітністю, зокрема прееклампсія) відіграють вирішальну роль у визначенні прогнозу для матері та плода.
Слід зазначити, що, за даними систематичних оглядів, поширеність прееклампсії в жінок із ЦД 1-го типу без вихідної діабетичної нефропатії перебуває в межах 9–17 %, але частота її значно зростає за умов супутньої діабетичної нефропатії та становить 35–66 % [4]. Останні десятиріччя ознаменувалися виявленням тісних взаємозв’язків між діабетичною нефропатією та прееклампсією. З одного боку, як було зазначено, діабетична нефропатія сприяє розвитку прееклампсії. З іншого боку, у жінок із ЦД 1-го типу навіть без супутньої діабетичної нефропатії наявність в анамнезі передчасних пологів та прееклампсії асоціюється з підвищеним ризиком термінальних стадій ниркової недостатності та смертності від відповідних причин у віддалені терміни їх життя. Цілком очікувано, що поширеність цього ускладнення зростає за умов вже наявних порушень функції нирок. Зокрема, згідно із даними численних досліджень, частота виникнення прееклампсії в жінок із хронічною хворобою нирок (у тому числі діабетичною нефропатією) позитивно корелює із рівнем креатиніну до вагітності: при сироватковій концентрації креатиніну < 125 мкмоль/л поширеність цього ускладнення становить 22 %, при рівні 125–180 мкмоль/л — 40 %, > 180 мкмоль/л — 60 %, а в пацієнток, які перебувають на хронічному діалізі, — 75 % [5, 11]. Крім того, іншими важливими факторами, що сприяють розвитку прееклампсії в жінок із діабетичною нефропатією, є такі: реєстрація мікроальбумінурії (МАУ), а тим більше протеїнурії в межах нефротичних значень та наявна на початку вагітності АГ [3]. Також важливо зазначити, що близько 45 % матерів із діабетичною нефропатією потребують передчасного розродження до 34 тижнів вагітності через виникнення прееклампсії. Усе це підкреслює необхідність та важливість прогнозування розвитку гіпертензивних розладів (та, зокрема, прееклампсії) у вагітних жінок із діабетичною нефропатією, що дозволить вчасно застосувати відповідні профілактичні заходи та більш ретельний моніторинг та врешті-решт сприятиме покращенню материнських та перинатальних наслідків вагітності у вказаній популяції жінок. Слід зазначити, що сьогодні важливе значення в прогнозуванні пре–еклампсії відводиться генетичним маркерам.
Мета дослідження: на підставі вивчення клініко-лабораторних та генетичних показників (профіль носійства поліморфних варіантів генів, характер міжгенних та генно-середовищних взаємодій) визначити фактори та їх поєднання, що дозволять прогнозувати розвиток прееклампсії у вагітних із цукровим діабетом 1-го типу та супутньою діабетичною нефропатією.
Матеріали та методи дослідження
Представлене дослідження було проведено за принципом «випадок — контроль» із включенням ретро- та проспективних даних. Відбір учасників дослідження відбувався з числа пацієнток, які в період з 2010 по 2014 рік знаходилися на стаціонарному лікуванні та/або були розроджені у відділенні акушерської ендокринології та вад розвитку плода ДУ «Інститут педіатрії, акушерства і гінекології НАМН України». З цією метою попередньо було проведено ретроспективний аналіз 460 історій пологів жінок із ЦД 1-го типу на предмет їх відповідності критеріям включення/виключення до дослідження. Критеріями включення пацієнток до дослідження були такі: наявність в анамнезі ЦД 1-го типу незалежно від наявності/відсутності супутньої діабетичної нефропатії (класи В–Т за класифікацією White в модифікації Pedersen); розвиток прееклампсії впродовж теперішньої вагітності (або її відсутність у пацієнток групи порівняння); одноплідна вагітність; надання письмової інформованої згоди на участь у дослідженні. Пацієнтки виключались із дослідження за наявності будь-якого з таких станів: хронічна АГ до або під час вагітності; неповний міхуровий занос (за даними патоморфологічного або ультразвукового дослідження); багатоплідна вагітність; паління; бажання пацієнтки перервати свою участь на будь-якому з етапів його проведення.
Прееклампсію діагностували відповідно до універсальних критеріїв для пацієнток із ЦД 1-го типу, які були нами заздалегідь розроблені на підставі Наказу № 676 МОЗ України, а також відповідних протоколів Американського коледжу акушерів-гінекологів (ACOG), Королівського коледжу акушерів-гінекологів (RCOG), Канадського товариства акушерів-гінекологів (SOGC) та Національного інституту здоров’я та якості медичної допомоги Великої Британії (NICE) (табл. 1).
Діагноз встановлювався за наявності основного в поєднанні з хоча б одним із допоміжних критеріїв. Діагноз діабетичної нефропатії встановлювали на підставі загальноприйнятних критеріїв C.E. Mogensen та співавт., згідно з цією класифікацією також визначалася стадія нефропатії в першому триместрі вагітності.
За результатами попереднього ретроспективного аналізу 41 пацієнтці було відправлено інформаційний лист — запрошення до участі в дослідженні та після отримання письмової згоди — індивідуальну паперову картку-бланк типу 903® (виробництво фірми Whatman, Німеччина), а також докладну інструкцію щодо техніки забору та підготовки зразків крові, термінів та умов їх відправлення до лабораторії. Картки із висушеними плямами крові транспортувались до лабораторії в пакетах типу Zip-Lock, що захищали їх від надмірної вологості. Після проходження ретельного контролю якості зразки допускались до використання в подальших етапах молекулярно-генетичного дослідження. У 19 жінок забір біологічного матеріалу для виконання молекулярно-генетичного дослідження відбувався безпосередньо під час їх перебування у відділенні. З цією метою забір зразків цільної периферійної крові виконувався в моновети об’ємом 2,7 мл із 3% розчином калієвої солі етилендіамінтетраоцтової кислоти як антикоагулянт із розрахунку 1 : 20 (Sarstedt, Німеччина). Моновети із зразками крові зберігалися при температурі –20 °С до моменту транспортування в лабораторію.
Пацієнток із ЦД 1-го типу, які були включені до дослідження, умовно розподілили на дві групи залежно від наявності/відсутності прееклампсії під час теперішньої вагітності. До основної групи увійшли 30 жінок із ЦД 1-го типу та клінічними проявами прееклампсії під час вагітності, до групи порівняння — 30 пацієнток із ЦД, у яких вагітність перебігала без прееклампсії. Усі жінки впродовж трьох триместрів вагітності спостерігались та отримували лікування, передбачене поточними нормативними документами.
Молекулярно-генетичні дослідження виконувались на базі лабораторії ДЗ «Референс-центр з молекулярної діагностики Міністерства охорони здоров’я України»; до того ж вивчалися 7 поліморфних варіантів 5 генів: А1166С поліморфізм гена рецептора 1-го типу ангіотензину II (AT2R1); C108T поліморфізм гена параоксонази 1 (PON-1); Thr83Ala та T138C поліморфізми гена матриксного Gla-протеїну (MGP); 4b/4a та G894T поліморфізми гена ендотеліальної NO-синтази (eNOS), а також інсерційно-делеційний (I/D) поліморфізм гена ангіотензинперетворюючого ферменту (ACE). Геномну ДНК виділяли з відібраних зразків крові за допомогою комерційного набору «ДНК-сорб-В».
Поліморфний варіант 4b/4a гена eNOS, а також I/D поліморфізм гена АСЕ визначали методом алель-специфічної полімеразної ланцюгової реакції (ПЛР) із використанням модифікованих методик. Для визначення А1166С поліморфізму гена AT2R1; C108T поліморфізму гена PON-1; Thr83Ala та T138C поліморфізмів гена MGP, а також G894T поліморфізму гена eNOS користувались методикою, що передбачала виконання ПЛР із подальшим аналізом поліморфізму довжини рестрикційних фрагментів.
Статистична обробка отриманих у дослідженні даних виконувалась з використанням парного двовибіркового t-тесту Стьюдента, критерію хі-квадрат (χ2) Пірсона, а також із розрахунком відношення шансів (ВШ) та 95% довірчого інтервалу за допомогою пакета програм SPSS 17.0. Для оцінки відносної якості окремо взятої статистичної моделі для заданого набору даних стосовно інших моделей розраховувався інформаційний критерій Акаїке (AIC). В окремих видах аналізу як метод моделювання була обрана бінарна логістична регресія. Характер міжгенних та ген-факторних взаємодій вивчався методом MDR за допомогою пакета програм MDR 2.0. У всіх видах аналізу відмінності вважалися статистично значущими при p < 0,05.
На проведення роботи було одержано дозвіл Комітету з медичної етики ДУ «Інститут педіатрії, акушерства і гінекології НАМН України».
Результати дослідження
Пацієнтки основної групи та групи порівняння суттєво відрізнялися за частотою поширеності діабетичної нефропатії. Даний стан був діагностований в 23 пацієнток основної групи (76,66 %) та в 13 пацієнток групи порівняння (36,66 %); подібні відмінності виявилися статистично значущими (χ2 = 8,21; p < 0,01; ВШ = 5,68; 95% ДІ 1,84–17,49).
З метою виявлення клінічних маркерів, що б могли асоціюватися з розвитком прееклампсії у вагітних із діабетичною нефропатією, на першому етапі дослі–дження нами були проаналізовані базові клінічні характеристики пацієнток основної групи залежно від наявності або відсутності діабетичної нефропатії в першому триместрі (табл. 2).
При цьому було встановлено різницю за стажем захворювання на ЦД 1-го типу (табл. 1): серед вагітних основної групи із діабетичною нефропатією стаж захворювання був суттєво тривалішим порівняно із вагітними без діабетичної нефропатії (відповідно 14,17 ± 0,93 року та 8,22 ± 2,69 року; p < 0,05).
Крім того, у пацієнток основної групи з діабетичною нефропатією спостерігалося вірогідне підвищення частоти ретинопатії (82,61 %) порівняно з вагітними основної групи без нефропатії (14,28 %) (χ2 = 8,41; p = 0,004; ВШ = 28,50; 95%ДІ 2,65–306,64). Слід зауважити, що поєднання діабетичної нефропатії та ретинопатії є досить поширеним. Наприклад, у дослідженні G.B. Piccolli та співавт. [8] у всіх випадках тяжкої діабетичної нефропатії було діагностовано також ретинопатію.
Поширеність інших діабетичних ускладнень не відрізнялася між пацієнтками основної групи з діабетичною нефропатією та без нефропатії. Отже необхідними чинниками розвитку прееклампсії на тлі вже наявної діабетичної нефропатії були тривалий стаж захворювання (понад 14 років) та поєднання нефропатії з наявною в першому триместрі вагітності діабетичною ретинопатією.
Для з’ясування впливу генетичних чинників на ризик виникнення прееклампсії в подальшому ми проаналізували та порівняли профіль поліморфних варіантів генів у пацієнток основної групи з наявною або відсутньою діабетичною нефропатією (табл. 3).
Як видно з табл. 3, у вагітних з існуючою на початок вагітності діабетичною нефропатією спостерігалася тенденція до зниження частоти поширення АА-генотипу за геном AT2R1, тоді як частота поширення АС-генотипу за вказаним геном була, навпаки, підвищеною порівняно з вагітними жінками без вже наявної нефропатії.
Подібна тенденція до зниження частоти більш поширених у популяції генотипів спостерігалася також для поліморфних варіантів гена eNOS. Зокрема, у пацієнток із діабетичною нефропатією 4b/4b-генотип за поліморфізмом 4b/4a та GG-генотип за поліморфізмом G894T виявлялися з частотою 60,87 та 69,57 %. Для порівняння, у пацієнток без діабетичної нефропатії ті ж самі генотипи реєструвалися з частотою 100 та 71,43 %. Слід зазначити, що частота поширення генотипів 4b/4a та 4a/4a за геном eNOS переважала серед жінок із наявною діабетичною нефропатією, оскільки в пацієнток без нефропатії ці генотипи взагалі не виявлялися.
При дослідженні частоти поширення поліморфних варіантів гена MGP нами було встановлено, що генотип -138TT частіше виявлявся в жінок із діабетичною нефропатією, а частота поширення гетерозиготного варіанта цього гена (-138ТС) за наявності діабетичної нефропатії була зниженою порівняно з жінками без діабетичної нефропатії. Генотип -138СС за вказаним геном взагалі не виявлявся у вагітних із діабетичною нефропатією. Узагальнюючи, слід зазначити, що частота поширеності згаданих генотипів -138ТС та -138СС (домінантна модель) значуще розрізнялася у вагітних основної групи з діабетичною нефропатією та без неї (χ2 = 6,41; p = 0,03; ВШ = 0,11; 95% ДІ 0,01–0,77) за рахунок зростання їх поширеності у вагітних без діабетичної нефропатії.
Після встановлення цих особливостей нами було проведено аналіз поширеності поліморфних варіантів досліджених генів у вагітних без діабетичної нефропатії в основній групі та групі порівняння для з’ясування питання, чи не є згадані поліморфні варіанти гена MGP та поліморфізм інших генів факторами ризику виникнення прееклампсії у вагітних із ЦД 1-го типу без існуючої на початок вагітності діабетичної нефропатії. Отримані нами результати наведені в табл. 4.
Як і у вагітних із вже наявною діабетичною нефропатією (табл. 3), у пацієнток без діабетичної нефропатії (табл. 4) значущими факторами ризику прееклампсії були генотипи ID та DD за геном ACE (χ2 = 5,78; p = 0,03; ВШ = 13; 95% ДІ 1,71–76,08), тоді як генотип II значуще знижував ризик розвитку прееклампсії (χ2 = 4,05; p = 0,044; ВШ = 0,08; 95% ДІ 0,01–0,79).
Вірогідне підвищення (χ2 = 3,89; p = 0,046; ВШ = 7,00; 95% ДІ 2,01–48,31) частоти поширення генотипу -138ТС за поліморфним варіантом Т138С гена MGP спостерігалося у вагітних основної групи без нефропатії (71,43 %) на відміну від пацієнток групи порівняння без нефропатії, у яких вказаний генотип виявлявся у 26,32 % випадків. Частота поширення генотипів -138СС була майже однаковою в цих пацієнток. За допомогою інформаційного критерію Акаїке (AIC = 11,12) вдалося встановити, що найбільш значущою моделлю в ризику розвитку прееклампсії у вагітних із ЦД 1-го типу була наддомінантна, за якою наявність генотипу -138ТС порівняно з генотипами -138ТТ та -138СС в 7 разів (ВШ = 7,00; 95% ДІ 1,13–61,57) підвищувала ризик розвитку прееклампсії в групі жінок без діабетичної нефропатії.
У вагітних групи порівняння без діабетичної нефропатії вірогідно частіше зустрічався генотип 4b/4b за геном eNOS (χ2 = 4,26; p = 0,035) порівняно з вагітними основної групи без діабетичної нефропатії.
Ураховуючи встановлений нами провідний вплив генів MGP та ACE на ризик виникнення прееклампсії, було вирішено вивчити поширеність деяких комбінацій генотипів за поліморфними варіантами вказаних генів в обох групах спостереження (незалежно від наявної або відсутньої діабетичної нефропатії).
/20.jpg)
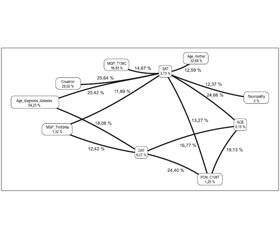
Серед вагітних основної групи було виявлено підвищену сумарну частоту поширення вищезгаданих комбінацій генотипів, що значуще різнилася із групою спостереження (χ2 = 6,79; p = 0,018; ВШ = 4,13; 95% ДІ 1,39–12,27). Отримані нами дані свідчать про те, що незалежно від наявності або відсутності вже існуючої діабетичної нефропатії наявність генотипів Thr83Ala та -138TC за геном MGP у поєднанні з генотипами DD та ID за генами ACE підвищує ризик розвитку прееклампсії у вагітних із ЦД 1-го типу. Наявність подібних комбінацій генотипів спонукала нас до вивчення ролі міжгенних взаємодій у зростанні ризику розвитку прееклампсії саме у вагітних основної групи з існуючою до початку вагітності діабетичною нефропатією (рис. 1).
/20_2.jpg)
Як видно з наведеного зображення, у розвитку прееклампсії в згаданих пацієнток провідну роль відіграє взаємодія між генами ACE та PON1 (виділено червоним кольором), а також між MGP (Thr83Ala) та eNOS (G894T) (виділено помаранчевим кольором). Показники ентропії для генів ACE та PON1 були вкрай невисокими, але за наявної взаємодії зростали на 19,14 %, так само як і для генів MGP (Thr83Ala) та eNOS (G894T), для яких під час аналізу міжгенної взаємодії було виявлено зростання на 7,51 %.
Отже, враховуючи найбільше зростання взаємного показника ентропії, саме комбінацію генів ACE та PON1 слід розглядати як найбільш значущу в прогнозуванні ризику розвитку прееклампсії у вагітних із ЦД 1-го типу та супутньою діабетичною нефропатією. Оскільки роль інсерційно-делеційного поліморфізму гена ACE у розвитку прееклампсії у вказаній популяції вагітних жінок було підтверджено нашими попередніми дослідженнями [1], особливий інтерес ми виявляли до вивчення асоціації між поліморфними варіантами гена PON1 та вірогідністю виникнення прееклампсії.
Щоб встановити, чи не підсилює обраний нами поліморфізм гена PON1 вплив діабетичної нефропатії на зростання ризику розвитку прееклампсії, ми користувалися методом бінарної логістичної регресії. До аналізу було залучено пацієнток обох груп спостереження (n = 60). Прогностична цінність отриманої нами моделі становила 68,3 %. До того ж було встановлено, що носійство 108СТ-генотипу за геном PON1 за умов наявності діабетичної нефропатії асоціювалось із зростанням ризику розвитку прееклампсії у 8 разів (p = 0,004; ВШ = 8,00; 95% ДІ 1,923–33,274). Генотип 108ТТ за геном PON1 мав незначущий вплив на розвиток прееклампсії в пацієнток із діабетичною нефропатією (p = 0,282; ВШ = 2,462; 95% ДІ 0,476–12,716).
Виявлена значуща відмінність була обумовлена різною частотою поширення генотипів за геном PON1 (C108T) у пацієнток із діабетичною нефропатією в основній групі та групі порівняння. Із 23 пацієнток із діабетичною нефропатією, які належали до основної групи, генотип 108СС було виявлено в 6 (26,09 %) жінок, генотип 108СТ — у 13 (56,52 %), генотип 108ТТ — у 4 (17,39 %). У групі порівняння розподіл генотипів серед 11 пацієнток із діабетичною нефропатією був таким: генотип 108СС було виявлено в 5 (45,45 %) осіб, генотип 108СТ — у 3 (27,27 %), генотип 108ТТ — у 3 (27,27 %). Розподіл генотипів розрізнявся між групами спостереження, найбільш значуще — тільки для гетерозиготного генотипу 108СТ. Отже, у 13 (43,33 %) із 30 вагітних основної групи виявляли діабетичну нефропатію в поєднанні з генотипом 108СТ, а в групі порівняння подібна комбінація спостерігалася лише в 3 (10 %) із 30 вагітних.
Ураховуючи значущу роль C108T поліморфізму гена PON1 у комбінації з діабетичною нефропатією в зростанні ризику розвитку прееклампсії під час вагітності в пацієнток із ЦД 1-го типу, а також встановлену методом MDR роль міжгенної взаємодії між поліморфними варіантами генів ACE та PON1 у розвитку прееклампсії у вказаній популяції пацієнток, у подальшому нами була проаналізована поширеність генотипів за ID поліморфізмом гена ACE у пацієнток із діабетичною нефропатією з урахуванням особливостей генотипу за геном PON1 (С108T). В обох групах спостереження у жодної пацієнтки із діабетичною нефропатією не було виявлено комбінації генотипів ACE(II)/PON1 (108TT). У 4 (100 %) пацієнток групи порівняння було виявлено комбінацію генотипів ACE(II)/PON1(108CC), а в основній групі ця комбінація була в однієї (25 %) пацієнтки, на відміну від комбінації генотипів ACE(II)/PON1(108CT), що відмічалась у трьох (75 %) пацієнток. Отримані нами відмінності не були значущими завдяки числу залучених до цього аналізу пацієнтів, але вони вказують на те, що в пацієнток із ЦД 1-го типу та супутньою нефропатією за наявності протективного II-генотипу за геном ACE розвиток прееклампсії може бути пов’язаним саме з несприятливим 108СТ за геном PON1. Тому аналіз поліморфізму гена PON1 є перспективним для обстеження жінок, у яких відсутні прогностично несприятливі поліморфні варіанти гена ACE.
При дослідженні ген-факторної взаємодії нами було проаналізовано понад 65 клініко-лабораторних показників та поліморфні варіанти досліджуваних генів. На рис. 2 наведені лише ті чинники, для яких було виявлено позитивну взаємодію.
З рис. 2 видно, що з урахуванням ген-факторної взаємодії провідне місце у виникненні прееклампсії в жінок із діабетичною нефропатією належить таким факторам: віку встановлення діагнозу ЦД 1-го типу та віку пацієнток на момент настання теперішньої вагітності у поєднанні з більш високими цифрами систолічного АТ у межах нормальних значень. Для показника діастолічного АТ у ризику розвитку прееклампсії спостерігали адитивний ефект із віком установлення діагнозу ЦД 1-го типу та поліморфними варіантами генів Thr83Ala_MGP, I/D_ACE, С108T_PON1. Останній факт підкреслює переважний вплив генетичного фактора саме на рівень діастолічного АТ, тобто він є провідним показником, що визначає тяжкість перебігу прееклампсії.
Обговорення
У результаті проведеного дослідження встановлені генетичні маркери, що дозволяють спрогнозувати розвиток прееклампсії в пацієнток із ЦД 1-го типу та супутньою діабетичною нефропатією без АГ. Підтверджено роль поліморфних варіантів генів ангіотензинперетворюючого ферменту (ACE), параоксонази-1 (PON1) та матриксного Gla-протеїну (MGP).
Слід зауважити, що попередніми роботами значущу роль у розвитку прееклампсії з числа зазначених генів вірогідно підтверджено лише для інсерційно-делеційного поліморфізму гена ACE. Проте в переважній більшості подібних досліджень ЦД виступав як критерій виключення, що врешті-решт не дозволяє порівняти результати нашого дослідження із даними, отриманими раніше іншими авторами. У представленому дослідженні наявність D-алелі в генотипі вагітних жінок із ЦД 1-го типу та супутньою прееклампсією асоціювалась із збільшеним ризиком виникнення прееклампсії, а II-генотип, навпаки, демонстрував протективні властивості. Більшість авторів, які провели схожі дослідження в різноманітних етнічних популяціях жінок без вже існуючого ЦД, теж сходяться на думці щодо наявності тісного взаємозв’язку між існуючою D-аллелю в генотипі вагітної та ймовірністю виникнення розвитку прееклампсії. Вважається, що D-алель асоціюється із підвищеною концентрацією ангіотензинперетворюючого ферменту та С-реактивного білка в сироватці крові; зниженою активністю реніну, а також компонентів антиоксидантного захисту в плазмі крові; порушеннями процесів вазодилатації, а також гістопатологічними змінами судин матки, тобто із факторами, що традиційно визнаються ключовими в патогенезі прееклампсії. У контексті поданої роботи слід також зазначити результати нашого попереднього дослідження [1], в якому в популяції вагітних жінок українського походження з ЦД 1-го типу (без зазначення наявності або відсутності діабетичних ускладнень та, зокрема, нефропатії) також доведено роль інсерційно-делеційного поліморфізму гена АСЕ в розвитку прееклампсії, а ID-генотип входив до складу найбільш значущих комбінацій генотипів, що асоціювались із розвитком ранніх форм прееклампсії.
Щодо поліморфного варіанта С108Т гена параоксонази-1 (PON1), то в наявних літературних джерелах взагалі відсутні дані щодо вивчення його ролі в розвитку прееклампсії. Однак існують окремі дослідження, в яких підтверджено асоціацію між іншими двома поліморфними варіантами цього гена (Q192R та L55M) та розвитком прееклампсії в загальній популяції пацієнток, які не страждають від ЦД 1-го типу. Можлива участь даного поліморфізму в патогенезі прееклампсії підтверджується тим фактом, що СС-генотип корелює із зниженим рівнем активності ферменту параоксонази-1 та, як наслідок, із поглибленням патологічних змін ліпідного профілю пацієнток та порушенням механізмів антиоксидантного захисту. Все це призводить до формування особливого ліпідного профілю у вагітних жінок, що сприяє розвитку прееклампсії.
Особливої уваги заслуговує підтвердження провідної ролі поліморфних варіантів гена матриксного Gla-протеїну (MGP) у розвитку прееклампсії. При виборі панелі генів, що планувалося вивчати в нашому дослідженні, ми брали до уваги наявні в літературі факти щодо властивостей даного білка, а саме його спроможність регулювати внутрішньосудинний кальцієвий гомеостаз. Даний компонент є вітамін-К-залежним протеїном, що є найпотужнішим інгібітором мінералізації артеріальної стінки з усіх сьогодні відомих. Зв’язування іонів кальцію молекулою білка відбувається завдяки його Gla-групі, яка попередньо піддається реакції карбоксилювання за наявності вітаміну К. За умов дефіциту вітаміну К у кровообігу пацієнтів циркулюють високі концентрації декарбоксильованих (функціонально неактивних) форм MGP, не здатних зв’язувати іони кальцію; це призводить до їх накопичення в судинній стінці та асоціюється із розвитком патологічних станів серцево-судинної системи. Виявлене серед вагітних основної групи значуще переважання комбінацій каузативних генотипів за генами ACE та MGP дозволяє нам сформулювати нову гіпотезу патогенезу прееклампсії: наявність мутантних алелей за геном MGP призводить до зменшення секреції або утворення функціонально неспроможного (неактивного) матриксного Gla-протеїну, що, зі свого боку, може сприяти накопиченню іонів кальцію в судинній стінці. У результаті підвищення концентрації іонів кальцію в судинній стінці збільшується її чутливість до вазоконстрикторних ефектів гуморальних факторів, концентрація та активність яких змінюється під впливом наявних мутантних алелей за геном АСЕ.
При вивченні ролі генетичного фактора в прогнозуванні ризику розвитку прееклампсії в популяції вагітних жінок із ЦД 1-го типу та супутньою нефропатією цілком логічно постає таке запитання: чи не впливають генетичні фактори, пов’язані з основним захворюванням (у нашому випадку — із діабетичною нефропатією), на профіль поліморфних варіантів генів, що асоціюються із розвитком прееклампсії? Зокрема, у літературі обговорюються наявність взаємозв’язку між ризиком розвитку діабетичної нефропатії та наступними поліморфними варіантами генів — I/D_ACE та G894T_eNOS. Отже, виявлене в нашому дослідженні значуще переважання частоти мутантних алелей та генотипів за інсерційно-делеційним поліморфізмом гена ACE серед вагітних основної групи (в якій значно частіше виявлялися пацієнтки з діабетичною нефропатією), на нашу думку, може бути пов’язаним із двома причинами: по-перше, із більшою поширеністю тих самих алелей та генотипів серед пацієнтів із діабетичною нефропатією; по-друге, із деякою схожістю основних ланок патогенезу діабетичної нефропатії та прееклампсії, що можуть бути обумовлені єдиним генетичним фактором. Крім того, у літературі існують дані щодо впливу поліморфних варіантів гена MGP на перебіг хронічної хвороби нирок. Наявність окремих молекулярних варіантів цього гена у хворих із прогресуючим перебігом хронічної хвороби нирок асоціюється із більш швидким досягненням термінальних стадій ниркової недостатності та раннім виникненням потреби в замісній терапії, а також підвищеним показником смертності серед вказаних хворих завдяки патологічним змінам (кальцифікації) судин та розвитком серцево-судинної патології. Останній факт дозволяє хоча б частково пояснити, чому у вагітних із діабетичною нефропатією саме цей ген має важливе значення у виникненні такої системної судинної патології, як прееклампсія.
Основним обмежуючим фактором у нашому дослідженні слід визнати невелику чисельність груп спостереження, що пов’язано із надзвичайно рідкісним поєднанням вже існуючого ЦД 1-го типу та прееклампсії в загальній популяції вагітних жінок (0,05–0,15 %). Отже, необхідно проведення широкомасштабних когортних досліджень для підтвердження отриманих та виявлення нових даних із поставленого питання.
Висновки
1. У популяції пацієнток із цукровим діабетом 1-го типу та діабетичною нефропатією без артеріальної гіпертензії ризик виникнення прееклампсії зростає в 8 разів у разі наявності 108СТ-генотипу за геном PON1.
2. Незалежно від наявності супутньої діабетичної нефропатії значущим фактором ризику розвитку прееклампсії у вагітних із цукровим діабетом 1-го типу є присутність ID- та DD-генотипів за геном ACE; II-генотип має протективну дію та значуще знижує ризик розвитку прееклампсії.
3. За умов відсутності вихідної діабетичної нефропатії розвиток прееклампсії в пацієнток вказаної популяції асоціюється із генотипом -138ТС за геном MGP.
4. Взаємодія між генами ACE та PON1, а також між MGP (Thr83Ala) та eNOS (G894T) відіграє провідну роль у розвитку прееклампсії в пацієнток із цукровим діабетом 1-го типу та вже існуючою діабетичною нефропатією.
5. При проведенні аналізу ролі ген-факторної взаємодії в розвитку прееклампсії було встановлено, що рівень систолічного артеріального тиску модифікувався переважно клінічними факторами, наявними в пацієнток, а рівень діастолічного артеріального тиску — генетичним компонентом (тобто поліморфними варіантами генів, що вивчалися в нашому дослі–дженні, — Thr83Ala_MGP, I/D_ACE та C108T_PON1).
6. У пацієнток із цукровим діабетом 1-го типу та супутньою діабетичною нефропатією без артеріальної гіпертензії можна рекомендувати вивчення зазначених поліморфних варіантів генів для визначення ризику розвитку прееклампсії.
Автори засвідчують відсутність конфлікту інтересів.
Список литературы
1. Авраменко Т.В. Молекулярно-генетический анализ и прогнозирование риска развития преэклампсии у беременных с сахарным диабетом 1 типа / Т.В. Авраменко, А.В. Грибанов, Н.Г. Горовенко, З.И. Россоха // Репродуктивное здоровье. Восточная Европа. — 2015. — Т. 41, № 5. — С. 79-88.
2. Bramham K. Pregnancy in diabetes and kidney disease / K. Bramham, D. Rajasingham // Journal of Renal Care. — 2012. — Vol. 38, Suppl. 1. — P. 78-89.
3. Edipidis K. Pregnancy in women with renal disease. Yes or no? / K. Edipidis // Hippokratia. — 2011. — Vol. 15, Suppl. 1. — P. 8-12.
4. Gaugler-Senden I.P.M. Severe early-onset preeclampsia: short- and long-term clinical psychosocial and biochemical aspects: PhD Thesis / Ingrid Petra Maria Gaugler-Senden. — Rotterdam: Erasmus Universiteit Rotterdam, 2011. — 147 p.
5. Palma-Reis I. Renal disease and hypertension in pregnancy / I. Palma-Reis, A. Vais, C. Nelson-Piercy, A. Banerjee // Clinical Medicine. — 2013. — Vol. 13. — P. 57-62.
6. Piccoli G.B. Pregnancy and chronic kidney disease: a challenge in all CKD stages / G.B. Piccoli, R. Attini, E. Vasario et al. // Clin. J. Am. Soc. Nephrol. — 2010. — Vol. 5, Issue 5. — P. 844-855.
7. Piccoli G.B. Risk of adverse pregnancy outcomes in women with CKD / G.B. Piccoli, G. Cabiddu, R. Attini et al. // J. Am. Soc. Nephrol. — 2015. — Vol. 26, Issue 8. — P. 2011-2022.
8. Piccoli G.B. Severe diabetic nephropathy in type 1 diabetes and pregnancy – a case series / G.B. Piccoli, E. Tavassoli, C. Melluzza et al. // Rev. Diabet Stud. — 2013. — Vol. 10, Issue 1. — P. 68-78.
9. Singh R. Pregnancy in patients with chronic kidney disease: maternal and fetal outcomes / R. Singh, N. Prasad, A. Banka et al. // Indian J. Nephrol. — 2015. — Vol. 25, Issue 4. — P. 194-199.
10. Wiles K.S. Pre-pregnancy counselling for women with chronic kidney disease: a retrospective analysis of nine years’ experience / K.S. Wiles, K. Bramham, A. Vais et al. // BMC Nephrol. — 2015. — Vol. 16, Issue 28. — doi: 10.1186/s12882-015-0024-6.
11. Williams D. Chronic kidney disease in pregnancy / D. Williams, J. Davison // Pregnancy Plus. — 2008. — Vol. 336, Issue 7637. — P. 211-215.
1. Avramenko TV, Hrybanov AV, Rossokha ZI. [Molecular and genetic analysis and prediction the risk for preeclampsia development in pregnant women with type 1 diabetes]. Reprodukty’vnoe zdorov’e. Vostochnaya Evropa. 2015;41(5):79-88. Russian.
2. Bramham K, Rajasingham D. Pregnancy in diabetes and kidney disease. Journal of Renal Care. 2012; 38 (Suppl. 1):78-89. doi: 10.1111/j.1755-6686.2012.00270.x.
3. Edipidis K. Pregnancy in women with renal disease. Yes or no? Hippokratia. 2011;15 (Suppl. 1):8–12. PMC3139682
4. Gaugler-Senden I. P. M. Severe early-onset preeclampsia: short- and long-term clinical psychosocial and biochemical aspects: PhD Thesis. Rotterdam: Erasmus Universiteit Rotterdam; 2011. 147 p.
5. Palma-Reis I, Vais A, Nelson-Piercy C, Banerjee A. Renal disease and hypertension in pregnancy. Clinical Medicine. 2013;13(1):57-62. PMID: 23472497
6. Piccoli GB, Attini R, Vasario E, Conijn A, Biolcati M, D’Amico F, Consiglio V, Bontempo S, Todros T. Pregnancy and chronic kidney disease: a challenge in all CKD stages. Clin J Am Soc Nephrol. 2010; 5(5):844-855. doi: 10.2215/CJN.07911109
7. Piccoli GB, Cabiddu G, Attini R, Vigotti FN, Maxia S, Lepori N, Tueveri M, Massidda M, Marchi C, Mura S, Coscia A, Biolcati M, Gaglioti P, Nichelatti M, Pibiri L, Chessa G, Pani A, Todros T. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol. 2015; 26(8):2011-2022. doi: 10.1681/ASN.2014050459
8. Piccoli GB, Tavassoli E, Melluzza C, Grassi G, Monzeglio C, Donvito V, Leone F, Attini R, Ghiotto S, Clari R, Moro I, Fassio F, Parisi S, Pilloni E, Vigotti FN, Giuffrida D, Rolfo A, Todros T. Severe diabetic nephropathy in type 1 diabetes and pregnancy – a case series. Rev Diabet Stud. 2013;10(1):68-78. doi: 10.1900/RDS.2013.10.68
9. Singh R, Prasad N, Banka A, Gupta A, Bhadauria D, Sharma RK, Kaul A. Pregnancy in patients with chronic kidney disease: maternal and fetal outcomes. Indian J Nephrol. 2015; 25(4):194-199. doi:10.4103/0971-4065.145127
10. Wiles KS, Bramham K, Vais A, Harding KR, Chowdhury P, Taylor CJ, Nelson-Piercy C. Pre-pregnancy counselling for women with chronic kidney disease: a retrospective analysis of nine years’ experience. BMC Nephrol. 2015;16(28). doi: 10.1186/s12882-015-0024-6
11. Williams D, Davison J. Chronic kidney disease in pregnancy. Pregnancy Plus. 2008;336(7637):211-215.
/20_2.jpg)

/16.jpg)
/18.jpg)
/19.jpg)
/20.jpg)
/21.jpg)