Журнал «Почки» Том 7, №3, 2018
Применение иммунобиологических лекарственных препаратов на основе моноклональных антител в нефрологической практике
Резюме
При різних захворюваннях нирок успішно застосовується імунобіологічна терапія на основі моноклональних антитіл із різним механізмом дії. До таких лікарських засобів належать: ритуксимаб (взаємодія з рецептором CD20 і елімінування В-клітин), екулізумаб (інгібування розщеплення С5 комплементу), буросумаб (блокування фактора росту фібробластів FGF23), адалімумаб (анти-TNF-α агент), абатацепт (блокує костимуляцію Т-клітин шляхом зв’язування з CD80), фрезолімумаб (нейтралізація TGF-β). Ці лікарські засоби застосовуються при системних захворюваннях нирок, фокально-сегментарному гломерулосклерозі, вовчаковому нефриті, мембранозній нефропатії, при трансплатації нирки.
При различных заболеваниях почек успешно применяется иммунобиологическая терапия на основе моноклональных антител с различным механизмом действия. К таким лекарственным препаратам относятся: ритуксимаб (взаимодействие с рецептором CD20 и элиминирование В-клеток), экулизумаб (ингибирование расщепления С5 комплемента), буросумаб (блокирование фактора роста фибробластов FGF23), адалимумаб (анти-TNF-α агент), абатацепт (блокирует костимуляцию Т-клеток путем связывания с CD80), фрезолимумаб (нейтрализация TGF-β). Эти лекарственные препараты применяются при системных заболеваниях почек, фокально-сегментарном гломерулосклерозе, волчаночном нефрите, мембранозной нефропатии, трансплантации почки.
With various diseases of the kidneys, immunobiological monoclonal antibody-based therapy with a different mechanism of action is successfully used. These drugs include: rituximab (interaction with the CD20 receptor and elimination of B cells), eсulizumab (inhibition of C5 complement cleavage), burosumab (blocking fibroblast growth factor 23), adalimumab (anti-tumor necrosis factor α agent), abatacept (blocks co-stimulation of T cells by binding to CD80), fresolimumab (neutralization of transforming growth factor β). These drugs are used for systemic kidney diseases, focal segmental glomerulosclerosis, lupus nephritis, membranous nephropathy, kidney transplantation.
Ключевые слова
захворювання нирок; моноклональні антитіла; ритуксимаб; екулізумаб; буросумаб; адалімумаб; абатацепт; фрезолімумаб
заболевания почек; моноклональные антитела; ритуксимаб; экулизумаб; буросумаб; адалимумаб; абатацепт; фрезолимумаб
kidney diseases; monoclonal antibodies; rituximab; eculizumab; burosumab; adalimumab; abatacept; fresolimumab
Более 100 лет прошло с момента первого терапевтического использования антител для лечения дифтерии лауреатом Нобелевской премии по физиологии и медицине Е. Behring [1]. По выражению другого лауреата Нобелевской премии, P. Erhlich [2], эти антитела являются так называемой магической пулей, которая избирательно поражает очаги заболевания, возникшие в организме человека. Первые моноклональные антитела (mAbs) были получены только в 1975 г. G. Kohler и C. Milstein [3], а через 10 лет FDA (U.S. Food and Drug Administration) утвердило для использования в клинической практике препарат Muromonab-CD3 [4] (мышиные антитела), являющийся иммуносупрессантом и предназначенный для снижения острого отторжения у пациентов с трансплантацией органов. Прогресс медицины в эпоху персонализированной терапии открывает новые возможности использования mAbs для лечения широкого спектра заболеваний, таких как рак, воспалительные, аутоиммунные, инфекционные, сердечно-сосудистые заболевания и др. За последние 20 лет около 50 лекарственных препаратов (ЛП) на основе моноклональных антител утверждены FDA, более 300 ЛП находятся в стадии клинических исследований [5], рынок моноклональных антител в 2017 г. составил свыше 85 миллиардов долларов США (более половины выручки от продуктов белковой инженерии).
Первоначально для лечения людей использовались мышиные антитела, однако у пациентов часто происходило образование антимышиных антител, что приводило к реакции гиперчувствительности и, как следствие, к снижению эффективности. Для уменьшения иммуногенности были разработаны химерные, гуманизированные («очеловеченные») и полностью человеческие антитела (рис. 1).
В 1997 г. ВОЗ утвердила международные непатентованные названия (International Nonproprietary Names, INNs) происхождения антител (табл. 1). Название всех ЛП на основе моноклональных антител оканчиваются на -mab.
На современном этапе медицины в связи с развитием новой фазы терапии применение моноклональных антител в лечении заболеваний находится на первом плане [6]. После того как в 2002 г. были применены для лечения первые полностью человеческие моноклональные антитела (Adalimumab), индустрия производства mAb экспотенциально расширяется [7, 8].
Мышиные антитела
Использование мышиных антител, полученных по гибридомной технологии, в клинической терапии имеет свои ограничения, что связано с различиями в иммунной системе человека и грызунов. У 50 % пациентов с мышиными антителами происходило образование антимышиных антител. Они имеют короткое время полужизни, низкую эффективность и безопасность. Это приводит к неспособности выполнять эффекторные функции, за исключением некоторых особых обстоятельств [9]. Мышиные антитела проявляют умеренный эффект стимуляции цитотоксичности. Так, их действие вызывает аллергические реакции и анафилактический шок, являющиеся результатом продуцирования человеческих антител против мышиных [10–12]. Чтобы минимизировать иммуногенные эффекты мышиных mAbs в терапии у человека, иммуногенные компоненты удаляют с помощью различных подходов [13].
Химерные антитела
Химерные антитела являются особыми типами терапевтических антител, полученных комбинацией генетических ингредиентов от людей и мышей, где все константные домены обеих цепей антитела мыши заменены на константные домены иммуноглобулина человека. Они производятся путем обработки константной области человека и вариабельной области мышиных антител [14]. Эти моноклональные антитела состоят примерно из 65 % человеческих антител для минимизации риска нежелательных реакций. FDA одобрило некоторые препараты, основанные на химерных антителах, для использования в терапии и научных исследованиях. Номенклатура названия химерных mAbs заканчивается суффиксом -ximab- (Infliximab, Rituximab, Abciximab) [15].
Гуманизированные антитела
За последние несколько лет гуманизированные мышиные mAbs были одобрены в качестве терапевтических лекарственных средств [16]. Молекулы этих антител на 95 % человеческого происхождения. В гуманизированных mAbs только гипервариабельные участки, отвечающие за комплементарное взаимодействие с антигеном, имеют мышиное происхождение. Иногда они слабее связываются с антигенами, чем исходные мышиные моноклональные антитела [17]. Для увеличения аффинности связывания антиген — антитело применяют технологию hain-shuffling. Примером гуманизированных «очеловеченных» моноклональных антител, одобренных FDA, являются Daclizumab, Omalizumab, Alemtuzumab [18].
Полностью человеческие антитела
Производство человеческих моноклональных антител обычным гибридомным методом является относительно сложным процессом из-за стресса, связанного с поддержанием клеточной линии и гибридом человека. Это также не представляется возможным из-за иммунизации людей in vivo различными антигенами [19]. Однако методы для получения человеческих mAbs стали возможны благодаря экспрессии фрагментов антител или единичных клеточных вариабельных фрагментов (Fab или ScFv) у бактерий. Фрагменты антител могут отображаться нитевидными бактериофагами для скрининга библиотеки антител [20, 21]. Большинство этих препаратов были получены либо с использованием трансгенных мышей, либо методом «фагового дисплея», технология которого хорошо известна и широко применяется для производства новых, полностью человеческих моноклональных антител [22, 23]. В 2003 г. лекарственный препарат Humira® был первым ЛП для лечения ревматоидного артрита, соcтоящим полностью из человеческих моноклональных антител [24].
Лекарственные препараты, различающиеся по своей структуре и механизму действия на основе моноклональных антител, находят применение в нефрологической практике.
1. Rituximab (ритуксимаб)
Лекарственный препарат Rituximab (Rituxan®, MabThera®) представляет собой первое химерное моноклональное антитело против CD20, одобренное FDA в 1998 г. [25]. Первоначально этот препарат использовали для лечения рака, особенно при злокачественных новообразованиях В-клеток. Однако за последние годы применение ритуксимаба в клинической практике значительно расширилось. Теперь он используется для лечения аутоиммунных заболеваний, в том числе ревматоидного артрита, системной красной волчанки, ANCA-ассоциированного васкулита [26, 27]. В-клетки играют важную роль в патогенезе опосредованного антителами аутоиммунного заболевания в качестве предшественников клеток, секретирующих антитела. Они продуцируют цитокины, активируют Т-клетки и могут действовать как антигенпрезентирующие клетки [28]. Рецептор CD20 обнаружен на поверхности В-клеток на стадии пре-В-клеток [29], не экспрессируется в других тканях и не появляется на В-клетках после их дифференцировки (рис. 2).
/107-1.jpg)
Хотя функция СD20 до конца не известна, предполагается, что он может играть роль в переносе Са2+ через плазматическую мембрану, поддерживая тем самым внутриклеточную концентрацию Са2+, что приводит к активации В-клеток. Механизм действия ритуксимаба заключается во взаимодействии с рецептором CD20 и элиминировании В-клеток. Это реализуется за счет нескольких механизмов: комплементзависимой и антителозависимой клеточной цитотоксичности, а также индукции апоптоза (рис. 3).
Применение ритуксимаба при некоторых заболеваниях почек и пересадке почек представлено в табл. 2 [30].
Системная красная волчанка и волчаночный нефрит
Системная красная волчанка (СКВ) представляет собой мультисистемное аутоиммунное заболевание, характеризующееся образованием аутоантител и осаждением иммунных комплексов в различных органах, что приводит к многочисленным изменениям в иммунной системе. Лечение СКВ часто представляет собой сложную задачу, так как большинство пациентов не реагируют на лечение первой линии. У них отмечается рецидив после начальной клинической ремиссии. Для этих пациентов рекомендуются препараты второй линии, включая ритуксимаб. Одним из проявлений СКВ является волчаночный нефрит (ВН).
В 2010 г. в систематическом обзоре [31] были опубликованы данные о терапевтической роли ритуксимаба у взрослых пациентов с ВН. В исследование было включено 106 пациентов с ВН, средний возраст которых составлял 31,4 года. Биопсия почки показала тип III ВН у 15 (14 %) больных, тип IV у 57 (54 %), тип V у 11 (10 %) и смешанный мембранный ВН у 23 (22 %) пациентов. Ритуксимаб вводили по 1000 мг через 15 дней 59 пациентам (56 %), у остальных 47 пациентов (44 %) в дозе, рекомендованной для лечения лимфомы, — 375 мг/м2/неделю в течение 4 недель. Некоторые пациенты (27; 25 %) получали внутривенно метилпреднизолон в дополнение к ритуксимабу. Полный ответ определяли как нормализацию уровней креатинина и альбумина в сыворотке и содержание альбумина в суточной моче меньше 0,5 г. В исследовании отмечена частичная почечная ремиссия всех параметров почки. Ответ на лечение ритуксимабом в соответствии с типом волчаночного нефрита был в 79 случаях: 8 (8 %) — тип III, 26 (67 %) — тип IV, 4 (57 %) — тип V и 18 (78 %) — со смешанным мембрано-пролиферативным ВН. Самые высокие показатели ответа наблюдались у пациентов с III и IV типами ВН, а низкие — у пациентов с V типом, особенно с ассоциированным пролиферативным поражением. Анализ исследования подтверждает возможность использования ритуксимаба при тяжелых, рефрактерных случаях волчаночного нефрита.
ANCA — васкулит, ассоциированный с антителами к цитоплазме нейтрофилов
ANCA-ассоциированный васкулит, связанный с поражением почек, может проявляться в виде гранулематоза с полиангиитом Вегенера, микроскопического полиангиита и почечного васкулита. Это заболевание представляет собой быстро прогрессирующий гломерулонефрит, который характеризуется острой почечной недостаточностью, гематурией и протеинурией [32]. В 2010 г. опубликованы результаты сравнительного исследования действия ритуксимаба и циклофосфамида у пациентов с ANCA-ассоциированными васкулитами почек [33, 34]. Целью многоцентрового, рандомизированного, двойного слепого исследования с перекрестным дизайном RAVE (ритуксимаб в/в 375 мг/м2/неделю в течение 4 недель и циклофосфамид 2 мг/кг/день) было подтверждение эффективности ритуксимаба в сравнении с циклофосфамидом. Показано, что схема на основе ритуксимаба более эффективна, чем на основе циклофосфамида. Ритуксимаб как препарат первой линии может быть предпочтителен в случае нежелательного назначения циклофосфамида (тератогенность, канцерогенность, гепатотоксичность) [35].
Смешанная криоглобулинемия
Смешанная криоглобулинемия (СК) — это системный васкулит, характеризующийся отложением иммунных комплексов в кровеносных сосудах, содержащих моноклональные антитела IgM (ревматоидный фактор) и поликлональные IgG. Клинические проявления могут варьировать от триады Мельтцера (астения, артралгия, пурпура) до более серьезных неврологических поражений и заболеваний почек [36]. СК часто связана с вирусом гепатита С (HCV) и может воздействовать на почки как мембрано-пролиферативный гломерулонефрит [37]. В течение последних нескольких лет были проведены исследования, в которых показана роль ритуксимаба в лечении пациентов с криоглобулинемическим васкулитом [38–40]. Результаты данных исследований показывают, что ритуксимаб следует использовать у пациентов со смешанной криоглобулинемией, васкулитами, поражением кожи, периферической нейропатией и гломерулонефритом.
Мембранозная нефропатия
Мембранозная нефропатия (МН) является ведущей причиной нефротического синдрома у взрослых. Характеризуется отложением иммунных комплексов в субэпителиальном пространстве клубочков, что приводит к утолщению их базальной мембраны [41]. Если мембранозную нефропатию не лечить, то около 30–40 % пациентов достигают терминальной стадии хронической почечной недостаточности в течение 5–10 лет после установления диагноза [42]. Так как В-клетки играют решающую роль в патогенезе МН в связи с продуцированием аутоантител и презентирующего антигена, ритуксимаб был использован как лекарственный препарат для лечения мембранозной нефропатии. В небольших когортных исследованиях ритуксимаб-индуцированное истощение В-клеток ассоциировалось с уменьшением протеинурии и улучшением иммунопатологических изменений [43, 44].
В исследовании P. Ruggenenti [45] сообщается, что из 100 пациентов с МН и нефротическим синдромом, которых лечили ритуксимабом, у 27 пациентов наблюдалась полная ремиссия (снижение экскреции белка мочи до нормальных значений). Эффект лечения зависел от времени, и все пациенты к 4 годам достигли полной или частичной ремиссии (рис. 4).
Принимая во внимание отличный профиль безопасности ритуксимаба, его следует рассматривать как лекарственное средство, способное заменить токсичные препараты первой линии при лечении МН, а также применять у тех пациентов с нефротическим синдромом, которые не реагируют на консервативное лечение.
Болезнь минимальных изменений и фокально-сегментарный гломерулосклероз
Наиболее частой причиной развития нефропатического синдрома у детей является идиопатический нефротический синдром, который преимущественно состоит из синдрома нефротических минимальных изменений (СНМИ) и фокально-сегментарного гломерулосклероза [46]. Примерно 80 % педиатрических пациентов показывают болезнь минимальных изменений по результатам биопсии почек. Большинство этих детей реагируют на лечение стероидами, поэтому биопсию почек обычно не выполняют у пациентов со стероидчувствительным нефротическим синдромом. Очень часто для этих пациентов применяют лечение ритуксимабом [47–49]. В 2010 г. Gulati at al. [50] опубликовал результаты многоцентрового когортного исследования о роли ритуксимаба при лечении пациентов со стероидрезистентным нефротическим синдромом (СРНС) и стероидзависимым нефротическим синдромом (СЗРС). Лечение ритуксимабом было назначено 57 пациентам (33 — с СРНС и 24 — с СЗРС), рефрактерным к стандартной терапии. После 6 месяцев терапии ритуксимабом у 9 (27,2 %) пациентов с СРНС была отмечена полная ремиссия, у 7 (21,2 %) — частичная ремиссия и 17 (51,5 %) пациентов не ответили на терапию. Среди 24 пациентов с СЗРС ремиссия была отмечена у 20 (83,3 %). Это исследование показало, что ритуксимаб эффективен в индуцировании и поддержании ремиссии у значительной части пациентов со стероидрезистентным и стероидзависимым нефротическим синдромом.
Трансплантация почки
Трансплантация почки является выбором для пациента с терминальной стадией почечной недостаточности, поскольку она предлагает улучшение качества жизни и имеет преимущества перед традиционным диализом. При трансплантации почки ритуксимаб использовался для снижения анти-АВО или антител к HLA (human leucocyte antigen — лейкоцитарный антиген человека) для лечения острого опосредованного антителами отторжения и посттрансплантационного лимфопролиферативного заболевания [51, 52]. Основной причиной острого опосредованного антителами отторжения у пациентов, перенесших трансплантацию почки, является присутствие естественных антигрупповых антител. Однако за последнее десятилетие появилась новая антителоредуцирующая и иммуномодуляторная терапия, которая сыграла важную роль в развитии техники десенсибилизации, используемой в модуляции анти-HLA и анти-групповых антител крови. При этом используют внутривенный иммуноглобулин (IVIG) и ритуксимаб для истощения В-клеток, что помогает преодолеть иммунологические барьеры и сделать трансплантацию успешной [53–56].
2. Eculizumab (экулизумаб)
Eculizumab (Soliris®, Alexion Pharmaceuticals Inc., Cheshire, Connecticut, USA) представляет собой гуманизированные моноклональные антитела, одобрен FDA в сентябре 2011 г. для лечения атипичного гемолитического уремического синдрома [57]. Моноклональными антителами является иммуноглобулин Ig2/4 каппа, состоящий из константных областей человека и мышиных областей, определяющих комплементарность. Экулизумаб имеет молекулярный вес ~ 140 кДа и состоит из 2 тяжелых цепей (448 аминокислот) и 2 легких цепей (214 аминокислот). Механизм действия экулизумаба состоит в ингибировании расщепления С5 до С5а и С5b, предотвращая, таким образом, образование комплекса терминального комплемента С5b-9, что приводит к блокированию комплементзависимого клеточного лизиса [58] (рис. 5).
Экулизумаб в лечении атипичного уремического синдрома и трансплантации почки
Гемолитический уремический синдром (ГУС) является одной из тромботических микроангиопатий. Формирование тромбов в артериолах и капиллярах дает классическую триаду сиптомов ГУС: микроангиопатическая гемолитическая анемия, тромбоцитопения и острая почечная недостаточность. У детей ГУС часто связан с продромальной инфекцией. Наиболее распространенной формой является STEC-ГУС (STEC-шигатоксинпродуцирующая E.coli), которая и вызывает заболевание в 90 % случаев. Приблизительно 5–10 % случаев являются нетипичными. Атипичные формы ГУС (аГУС) встречаются в раннем возрасте. Исследования показали, что пациенты с ГУС имеют генетические дефекты, которые приводят к неправильной регуляции пути комплемента [59, 60].
В 2009 г. R. Gruppo и R. Rother [61] сообщили о первом использовании экулизумаба у пациента с врожденным аГУС. У 18-месячного мальчика (первые симптомы ГУС появились спустя 8 часов после рождения) было 3 рецидива. Лечение заключалось в использовании обменной трансфузии, инфузии плазмы и плазмафереза. На 35-й день четвертого рецидива был назначен экулизумаб в/в в дозе 300 мг еженедельно в течение 3 недель, а затем 600 мг каждые 2 недели. Гематологические параметры и функция почек начали улучшаться через 2 дня, а полная ремиссия была отмечена через 10 дней.
После этой публикации несколько авторов предоставили отчеты о клинических случаях, которые демонстрируют полезность использования экулизумаба для лечения аГУС. Так, в 2011 г. А. Lapeyraque и др. [62] описали использование экулизумаба у 7-летней девочки с ГУС, имеющей 2 фактора Н-мутации (S119L и V1197A). Лечение экулизумабом было начато в ответ на серьезное обострение, связанное с гипертонией и острой почечной недостаточностью. Схема лечения предусматривала 600 мг экулизумаба в неделю в течение 3 недель, а затем 600 мг каждые 2 недели. В течение первой недели нормализовалось давление крови, показатель тромбоцитов и функция почек.
Экулизумаб также использовался для лечения детей с аГУС, которые подверглись трансплантации почки. Известно, что аГУС прогрессирует до терминальной стадии почечной недостаточности у 50 % пациентов, а у пациентов с мутацией фактора Н риск отторжения аллотрансплантата почек в течение 2 лет достигает 80 %. В 2010 г. J. Davin [63] описал использование экулизумаба у 17-летней пациентки после трансплантации почки. Через 10 месяцев после трансплантации у нее развились симптомы снижения функции почек и аллергические реакции на обычную инфузию плазмы. Экулизумаб был назначен в стартовой дозе 900 мг еженедельно в течение 4 недель с последующей дозой 1200 мг каждые 2 недели. Спустя 6 месяцев креатинин сыворотки пациентки стабилизировался до 1,36 мг/дл и нормализовалось количество тромбоцитов.
3. Burosumab (буросумаб)
Burosumab (Сrysvita®), известный как KRN23 (производитель — компания Ultragenyx Pharmaceutical Inc. и Kyowa Hakki Kirin Co.Ltd., Япония), представляет собой человеческие моноклональные антитела (IgG1), предназначенные для лечения редкого генетического заболевания — Х-связанной гипофосфатемии (X-Linked Hypophosphataemia, XLH). Пациенты с XLH имеют мутации гена PHEX (фосфатрегулирующий ген с гомологией к эндопептидазам на Х-хромосоме), который изменяет систему контроля за фосфатом, приводит к хроническому истощению фосфата почки, гипофосфатемии и дефектам костной минерализации, проявляющейся как рахит и остеомаляция, а также может воздействовать на другие ткани. Препарат одобрен FDA в апреле 2018 г. для лечения взрослых и детей от 1 года с Х-сцепленной гипофосфатемией (редкой наследственной формой рахита) [64]. При этом заболевании происходит повышенная экскреция фосфора с мочой, что приводит к нарушению роста костей. Терапия витамином D в этом случае является неэффективной. Моноклональные антитела препарата буросумаб действуют на фактор роста фибробластов 23 (FGF23), который снижает уровень фосфатов в сыворотке путем почечной экскреции. Ингибирование чрезмерной активности FGF23 способствует увеличению тубулярной реабсорбции фосфатов и увеличению концентрации в сыворотке 1,25-гидроксивитамина D (рис. 6).
/109-2.jpg)
Рекомендуемое дозирование буросумаба в виде подкожной инъекции основано на весе педиатрических и взрослых пациентов.
1. Педиатрические пациенты (от 1 года до 18 лет):
— рекомендуемая начальная доза — 0,8 мг/кг массы тела, вводить каждые 2 недели;
— доза может быть увеличена до 2 мг/кг, вводить каждые 2 недели до достижения нормального содержания фосфора в сыворотке;
— измерение фосфора в сыворотке натощак каждые 4 недели в течение первых 3 месяцев лечения, а затем по мере необходимости. Уменьшить дозу препарата, если фосфор в сыворотке выше нормальных референтных значений.
2. Взрослые пациенты (18 лет и старше):
— рекомендуемая начальная доза — 1,0 мг/кг массы тела, вводить каждые 4 недели;
— измерение фосфора в сыворотке натощак каждые 4 недели в течение первых 3 месяцев лечения, а затем по мере необходимости. Уменьшить дозу препарата, если фосфор в сыворотке выше нормальных референтных значений.
Пример использования буросумаба у пациентов (дети 5–12 лет), получающих препарат каждые 2 недели, представлен на рис. 7.
4. Adalimumab (адалимумаб) и Infliximab (инфликсимаб)
Фактор некроза опухоли альфа (TNF-α) является основным провоспалительным цитокином при многих хронических воспалительных заболеваниях, включая ревматоидный артрит, ювенильный ревматоидный артрит (РА), анкилозирующий спондилоартрит, псориатический артрит и болезнь Крона [65]. Анти-TNFα-агенты, используемые в лечении РА и других ревматологических заболеваний, включают химерные моноклональные антитела Infliximab (Remicade®, Centocor Inc.) и полностью человеческие моноклональные антитела Adalimamub (Humira®, Abbott) (рис. 8).
У пациентов с РА возникают заболевания почек, которые чаще всего связаны с вторичным амилоидозом, гломерулонефритом, васкулитом почечных сосудов или осложнениями при применении лекарственной терапии [66]. Гломерулонефрит является наиболее распространенной патологией при РА с частотой 17–67 %. В некоторых исследованиях показана эффективность и безопасность анти-TNFα-агентов у пациентов с РА и почечной недостаточностью [67, 68]. Однако это были отдельные небольшие отчеты, которые не могут подтвердить безопасность конкретного анти-TNFα-агента. Имеется несколько сообщений об использовании адалимумаба при лечении пациентов с РА и почечной недостаточностью. S. Kobak [69] продемонстрировал эффективность и безопасность адалимумаба (лечение 12 недель) у 65-летнего мужчины с активным анкилозирующим спондилитом, который проходил перитонеальный диализ. Было отмечено, что адалимумаб не влияет на функцию почек и может безопасно вводиться даже пациентам на диализе, так как данный препарат гидролизуется в лизосомах, подобно другому анти-TNFα-агенту, такому как инфликсимаб [70]. Эти данные могут свидетельствовать о том, что адалимумаб может быть потенциальным терапевтическим препаратом для пациентов с РА и почечной недостаточностью, включая гемодиализ. Антагонисты TNF-α уменьшают протеинурию в модели повреждения почек, индуцированного ангиотензином II, и других моделях, таких, например, как ФСГС. В исследовании фазы I показано, что лечение адалимумабом в течение 16 недель способствовало стабилизации функции почек и уменьшению протеинурии у 10 пациентов со стероидустойчивой ФСГС [71].
5. Abatacept (абатацепт)
Abatacept (Orencia®, Bristol-Myers Squibb) является растворимым белком человека, состоящим из внеклеточного домена CTLA4 (Cytotoxic T-Lymphocyte Ag4), связанного с модифицированным (исключение активации комплемента) Fc-фрагментом человеческого Igl [72, 73]. Абатацепт (АБЦ) блокирует костимуляцию Т-клеток путем связывания с CD80/CD86, ингибирует взаимодействие с CD28 и подавляет последующие иммунные эффекторные механизмы (провоспалительные цитокины, аутоантитела). Модифицированный фрагмент в АБЦ неактивен и не связан с неблагоприятными событиями, вызванными зависимой от комплемента или антителозависимой клеточно-опосредованной цитотоксичностью. Селективное ингибирование активации Т-лимфоцитов приводит к блокированию синтеза провоспалительных цитокинов и клеток, которые вовлечены в иммунопатогенез аутоиммунных заболеваний (рис. 9).
Хотя абатацепт был разработан Bristol-Myers Squibb как лекарственный препарат для лечения ревматоидного артрита в случае неадекватного ответа на анти-TNFα-терапию, тем не менее он нашел применение в нефрологической практике [74].
В 2013 г. Yu С. et al. [75] опубликовали исследование лечения абатацептом пяти пациентов с фокально-сегментарным гломерулосклерозом. Терапия абатацептом индуцировала успешную частичную или полную ремиссию протеинурии, вызванную первичной или рекуррентной ФСГС, у всех 5 пациентов.
В 2 рандомизированных клинических исследованиях для лечения пролиферативного волчаночного нефрита оценивали эффективность применения абатацепта с микофенолата мофетилом и плацебо (NCT00430677), а также абатацепта с циклофосфамидом (NCT00774852).
6. Fresolimumab (фрезолимумаб)
Fresolimumab (Genzyme Corporation, Cambridge, MA) представляет собой человеческие моноклональныне антитела и предназначен для лечения фокально-сегментарного гломерулосклероза, рака (рак почки, меланома), идиопатического легочного фиброза.
Трансформирующий фактор роста бета (TGF-β) является мультифункциональным регулятором, который модулирует пролиферацию, дифференциацию, апоптоз, адгезию и миграцию различных типов клеток [76]. TGF-β млекопитающих включает три основные изоформы — TGF-β1, TGF-β2 и TGF-β3 [77].
В течение длительного времени TGF-β1 считался ключевым медиатором фиброза почек при активации сигнального Smad-пути. Недавно TGF-β также был признан регулятором функции подоцитов. При ФСГС у пациентов и в экспериментальных моделях было продемонстрировано увеличение экспрессии TGF-β в почках и моче. Сверхэкспрессия TGF-β в подоцитах приводит к подоцитопении и гломерулосклерозу. В культивируемых подоцитах TGF-β влияет на выживаемость клеток и вызывает изменение цитоскелета и адгезию клеток, аналогично в процессах in vivo. Эти результаты подтверждают, что целевая терапия TGF-β в почках может быть ренозащитной и оказывать благотворное влияние на тяжесть и прогрессирование ФСГС. Фрезолимумаб способен нейтрализовать все три формы TGF-β. Клинические исследования фазы I, которые проводились в 2011 г. H. Trachtman et al. [78] у 16 пациентов с первичным ФСГС и нефротической протеинурией, показали, что фрезолимумаб хорошо переносится и связан с уменьшением протеинурии.
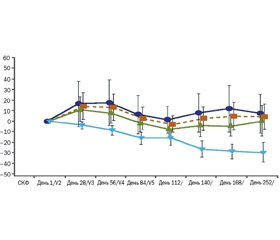
В фазе II двойного слепого, плацебо-контролируемого, рандомизированного исследования, выполненного F. Vincenti и др. [79] в 2017 г., оценивали действие фрезолимумаба у пациентов со стероидрезистентным первичным фокально-сегментарным гломерулосклерозом. Из 36 пациентов, включенных в исследование, 10 пациентов принимали плацебо, 14 пациентов — 1 мг/кг фрезолимумаба и 12 пациентов — 4 мг/кг фрезолимумаба. Исходная оценка скорости клубочковой фильтрации (СКФ) составляла 63 мл/мин/1,73 м2 и соотношение белок/креатинин в моче — 6190 мг/г. На 112-й день среднее процентное изменение соотношения белок/креатинин в моче было 18,5 % (1 мг/кг), 10,5 % (4 мг/кг) и 9 % (плацебо). Отмечено увеличение СКФ у пациентов, принимавших фрезолимумаб в дозе 1 и 4 мг/кг, по сравнению с группой плацебо (рис. 10).
Таким образом, фрезолимумаб может представлять собой терапию с новым механизмом действия при фиброзе почек и первичной ФСГС [80].
7. Epratuzumab (эпратузумаб)
Epratuzumab (Immunomedics, Inc., Morris Plains, NJ, USA) представляет собой гуманизированные моноклональные антитела, направленные против CD22, которые присутствуют на материнских В-клетках и на многих типах злокачественных В-клеток [81]. Введение эпратузумаба приводит к значительному снижению количества В-клеток. Эти моноклональные антитела используются для лечения лимфобластной лейкемии, неходжкинской лимфомы, системной красной волчанки.
При системной красной волчанке часто поражаются почки. Чаще всего это связано с аккумуляцией гломерулярных иммунных комплексов, которые поражают гломерулы и почечный тубулоинтерстициум. Если этот процесс не остановить, то может произойти хроническое рубцевание всей паренхимы почки. Волчаночный нефрит является проявлением тяжелой формы СКВ, а хроническое заболевание почек — результатом волчаночного нефрита. В современных схемах лечения используют неспеци–фические иммуносупрессанты, одним из которых может быть эпратузумаб.
Выводы
В последние годы в мире для лечения заболевания почек активно и успешно используют моноклональные антитела с различным механизмом действия (ингибиторы В-клеток, TNF-α, TGF-β, С5 комплемента, фактор роста фибробластов FGF23, CD80). Данные лекарственные препараты применяются при системных заболеваниях почек, фокально-сегментарном гломерулосклерозе, волчаночном нефрите, мембранозной нефропатии, трансплантации почки. На сегодня в Украине практическое использование этих ЛП ограничено их высокой стоимостью и официальной регистрацией (лишь некоторые из представленных лекарственных препаратов имеют разрешение для практического применения).
Конфликт интересов. Автор заявляет об отсутствии какого-либо конфликта интересов при подготовке данной статьи.
Список литературы
1. Behring E. Die praktischen Ziele der Blutserumtherapie, 1892.
2. Erhlich P. // Pysiology or medicine 1901–1921. Elsevier Publishing 1. Co., Amsterdam; 1967. P. 304-320.
3. Kohler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity // Nature. 1975; 256: 495-497.
4. Smith S.L. Ten years of Orthoclone OKT3 (muromonab-CD3): a review // Journal of transplant coordination: official publication of the North American Transplant Coordinators Organization (NATCO). 1996; 6 (3): 109-119; quiz 119-1. doi: 10.7182/prtr.1.6.3.814513u185493182.
5. Ecker D., Jones S., Levine H. The therapeutic monoclonal antibody market // Mabs. 2015; 7: 9-14. doi: 10.4161/19420862.2015.989042.
6. Wang S. Advances in the production of human monoclonal antibodies // Antibo Techn Journ. 2011; 1: 1-4.
7. Sheinfeld N. Adalimumab (Humira): a review // J. Drugs Dermatol. 2003 Aug; 2(4): 375-7.
8. Nelson A.L., Dhimolea E., Reichert J.M. Development trends for human monoclonal antibody therapeutics // Nat. Rev. Drug Discovery. 2010; 9(10): 767-774. doi: 10.1038/nrd3229.
9. Bernett M.J., Karki S.G., Moore G.L., Leung I.W.L., Chen H., Pong E., Nguyen D.H.T., Jacinto J., Zalevsky J., Muchhal U.S. et al. Engineering Fully Human Monoclonal Antibo–dies from Murine Variable Regions // J. Mol. Biol. 2010; 396(5): 1474-1490. doi: 10.1016/j.jmb.2009.12.046.
10. Ansar W., Ghosh S. Monoclonal Antibodies: A Tool in Clinical Research // Indian J. Clin. Med. 2013; 4: 9-21. doi: 10.4137/IJCM.S11968.
11. Li J., Zhu Z. Research and development of next generation of antibody-based therapeutics // Acta Pharmacol. Sin. 2010; 31(9): 1198-1207. doi: 10.1038/aps.2010.120.
12. Reff M.E., Hariharan K., Braslawsky G. Future of monoclonal antibodies in the treatment of hematologic malignancies // Cancer. Control. 2002; 9(2): 152-66. doi: 10.1177/107327480200900207.
13. Rodrigues M.E., Costa A.R., Henriques M., Azeredo J., Oliveira R. Technological progresses in monoclonal antibody production systems // Biotechnol. Prog. 2010; 26(2): 332-351. doi: 10.1002/btpr.348.
14. Lin W., Kurosawa K., Murayama A., Kagaya E., Ohta K. B-cell display-based one-step method to generate chimeric human IgG monoclonal antibodies // Nucleic. Acids. Res. 2011; 39(3): 1-10. doi: 10.1093/nar/gkq1122.
15. Mak T.M., Hanson B.J., Tan Y.J. Chimerization and characterization of a monoclonal antibody with potent neutralizing activity across multiple influenza A H5N1 clades // Antiviral. Res. 2014; 107(1): 76-83. doi: 10.1016/j.antiviral.2014.04.011.
16. Wang S. Advances in the production of human monoclonal antibodies // Antib. Technol. J. 2011; 1: 1-4. doi.org/10.2147/ANTI.S20195.
17. Chandel P., Harikumar S.L. Pharmaceutical monoclonal antibodies: Production, guidelines to cell engineering and applications // Int. J. Pharm. Sci. 2013; 5(2): 13-20.
18. Harding F.A., Stickler M.M., Razo J., DuBridge R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions // Mabs. 2010; 2(3): 256-265.
19. Bernett M.J., Karki S.G., Moore G.L., Leung I.W.L., Chen H., Pong E., Nguyen D.H.T., Jacinto J., Zalevsky J., Muchhal U.S. et al. Engineering Fully Human Monoclonal Antibo–dies from Murine Variable Regions // J. Mol. Biol. 2010; 396(5): 1474-1490. doi: 10.1016/j.jmb.2009.12.046.
20. Steinitz M. Human Monoclonal Antibodies // Methods in molecular biology (Clifton N.J.). 2014; 1060: 111-22.
21. Medecigo M., Manoutcharian K., Vasilevko V., Govezensky T., Munguia M.E., Becerril B., Luz-Madrigal A., Vaca L., Cribbs D.H., Gevorkian G. Novel amyloid-beta specific scFv and VH antibody fragments from human and mouse phage display antibody libraries // J. Neuroimmunol. 2010; 223(1): 104-114. doi: 10.1016/j.jneuroim.2010.03.023
22. Solforosi L., Mancini N., Canducci F., Clementi N., Sautto G.A., Diotti R.A., Clementi M., Burioni R. A phage display vector optimized for the generation of human antibody combinatorial libraries and the molecular cloning of monoclonal antibody fragments // New Microbiol. 2012; 35(3): 289-294.
23. Nelson A., Dhimolea E., Reichert J. Development trends for human monoclonal antibody therapeutics // Nature reviews drug discovery. 2010; 9: 767-774. doi: 10.1038/nrd3229.
24. Ahmad Z.A., Yeap S.K., Ali A.M., Ho W.Y., Alitheen N.B.M., Hamid M. ScFv antibody: Principles and clinical application // Clin. Dev. Immunol. 2012; 1-15. doi: 10.1155/2012/980250.
25. Leget G.A., Czuczman M.S. Use of rituximab, the new FDAapproved antibody // Curr. Opin. Oncol. 1998; 10: 548-551.
26. Edwards J.C., Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes // Rheumatology. 2001; 40: 205-211.
27. Turner-Stokes T., Lu T.Y., Ehrenstein M.R. et al. The efficacy of repeated treatment with B-cell depletion therapy in systemic lupus erythematosus: an evaluation // Rheumatology 2011; 50: 1401-1408. doi: 10.1093/rheumatology/ker018.
28. Kado R., Sanders G., McCune W.J. Suppression of normal immune responses after treatment with rituximab // Curr. Opin Rheumatol. 2016; 28: 251-258. doi: 10.1097/BOR.0000000000000272.
29. Seyfizadeh N., Seyfizadeh N., Hasenkamp J. et al. A molecular perspective on rituximab: a monoclonal antibody for B cell non Hodgkin lymphoma and other affections // Crit. Rev. Oncol. Hematol. 2016; 97: 275-290. doi: 10.1016/j.critrevonc.2015.09.001.
30. Ramanath V., Nistala R. & Chaudhary K. Update on the role of rituximab in kidney diseases and transplant // Expert opinion on biological therapy. 2011: 1-11. doi: 10.1517/14712598.2012.646984.
31. Ramos-Casals M., Diaz-Lagares C., Soto-Cardenas M.J. et al. Rituximab therapy in lupus nephritis: current clinical evidence // Clin. Rev. Allergy Immunol. 2011; 40: 159-69. doi: 10.1007/s12016-010-8205-3.
32. Walters G.D., Willis N.S., Craig J.C. Interventions for renal vasculitis in adults. A systematic review // BMC Nephrol. 2010; 11: published online 24 June 2010. doi: 10.1186/1471-2369-11-12.
33. Jones R.B., Tervaert J.W., Hauser T. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis // N. Engl. J. Med. 2010; 363: 211-20. doi: 10.1056/NEJMoa0909169.
34. Stone J.H., Merkel P.A., Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis // N. Engl. J. Med. 2010; 363: 221-32. doi: 10.1056/NEJMoa0909905.
35. Guerry M.-J.C.J., Brogan P., Bruce I.N. et al. Recommendations for the use of rituximab in anti-neutrophil cytoplasm antibody-associated vasculitis // Rheumatology. 2012; 51(4): 634-643. doi: 10.1093/rheumatology/ker150.
36. Monti G., Galli M., Invernizzi F. et al. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias // QJM. 1995; 88: 115-26.
37. Garini G., Allegri L., Lannuzzella F. et al. HCV-rela–ted cryoglobulinemic glomerulonephritis: implications of antiviral and immunosuppressive therapies // Acta Biomed. 2007; 78:
51-9.
38. Zaja F., De Vita S., Russo D. et al. Rituximab for the treatment of type II mixed cryoglobulinemia // Arthritis Rheum. 2002; 46: 2252-4; author reply 2254-2255. doi: 10.1182/blood-2002-09-2856.
39. Roccatello D., Baldovino S., Rossi D. et al. Rituximab as a therapeutic tool in severe mixed cryoglobulinemia // Clin. Rev. Allergy Immunol. 2008; 34: 111-17.
40. Zaja F., Russo D., Fuga G. et al. Rituximab for the treatment of type II mixed cryoglobulinemia // Haematologica. 1999; 84: 1157-8.
41. Ronco P., Debiec H. Antigen identification in membranous nephropathy moves toward targeted monitoring and new therapy // J. Am. Soc. Nephrol. 2010; 21: 564-9. doi: 10.1681/ASN.2009121220.
42. Ruggenenti P., Cravedi P., Remuzzi G. La–test treatment strategies for membranous nephropathy // Expert. Opin Pharmacother. 2007; 8: 3159-71. doi: 10.1517/14656566.8.18.3159.
43. Cravedi P., Sghirlanzoni M.C., Marasa M., Salerno A., Remuzzi G., Ruggenenti P. Efficacy and safety of Rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study // Am. J. Nephrol. 2011; 33: 461-8. doi: 10.1159/000327611.
44. Ruggenenti P., Cravedi P., Sghirlanzoni M.C., Gagliardini E., Conti S., Gaspari F. et al. Effects of Rituximab on morphofunctional abnormalities of membranous glomerulopathy // Clin. J. Am. Soc. Nephrol. 2008; 3: 1652-9. doi: 10.2215/CJN.01730408.
45. Ruggenenti P., Cravedi P., Chianca A., Perna A., Ruggiero B., Gaspari F. et al. Rituximab in idiopathic membranous nephropathy // J. Am. Soc. Nephrol. 2012; 23: 1416-25. doi: 10.1681/ASN.2012020181.
46. Van Husen M., Kemper M.J. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome // Pediatr. Nephrol. 2011; 26: 881-92. doi: 10.1007/s00467-010-1717-5.
47. Guigonis V., Dallocchio A., Baudouin V. et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases // Pediatr. Nephrol. 2008; 23: 1269-79. doi: 10.1007/s00467-008-0814-1.
48. Kamei K., Ito S., Nozu K. et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children // Pediatr. Nephrol. 2009; 24: 1321-8.
49. Prytula A., Iijima K., Kamei K. et al. Rituximab in refractory nephrotic syndrome // Pediatr. Nephrol. 2010; 25: 461-8. doi: 10.1007/s00467-009-1376-6.
50. Gulati A., Sinha A., Jordan S.C. et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report // Clin. J. Am. Soc. Nephrol. 2010; 5: 2207-12.
51. Fuchinoue S., Ishii Y., Sawada T. et al. The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction // Transplantation. 2011; 91: 853-7.
52. Becker Y.T., Becker B.N., Pirsch J.D., Sollinger H.W. Rituximab as treatment for refractory kidney transplant rejection // Am. J. Transplant. 2004; 4: 996-1001. doi: 10.1111/j.1600-6143.2004.00454.x.
53. Imamura R., Ishiguro S., Shi Y. et al. ABO-incompatible kidney transplantation with anti-CD20 monoclonal antibodies, intravenous immunoglobulin and plasmapheresis without splenectomy: a case report // Xenotransplantation. 2006; 13: 133-5.
54. Sonnenday C.J., Warren D.S., Cooper M. et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy // Am. J. Transplant. 2004; 4: 1315-22. doi: 10.1111/j.1600-6143.2004.00507.x.
55. Tyden G., Kumlien G., Genberg H. et al. ABO incompa–tible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab // Am. J. Transplant. 2005; 5: 145-8. doi: 10.1111/j.1600-6143.2004.00653.x.
56. Toki D., Ishida H., Horita S. et al. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients // Transpl. Int. 2009; 22: 447-54. doi: 10.1111/j.1432-2277.2008.00821.x.
57. Davis J. Eculizumab // Am. J. Health Syst. Pharm. 2008; 65: 1609e15.
58. Rother R.P., Rollins S.A., Mojcik C.F. et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria // Nat. Biotechnol. 2007; 25: 1256-1264. doi: 10.1038/nbt1344.
59. Scheiring J., Rosales A., Zimmerhackl L.B. Clinical practice: today’s understanding of the haemolytic uraemic syndrome // Eur. J. Pediatr. 2010; 169: 7-13.
60. Waters A.M., Licht C. aHUS caused by complement dysregulation: new therapies on the horizon // Pediatr. Nephrol. 2011; 26: 41-57. doi: 10.1007/s00467-010-1556-4.
61. Gruppo R.A., Rother R.P. Eculizumab for congenital aty–pical hemolytic-uremic syndrome [letter] // N. Engl. J. Med. 2009; 360: 544-6. doi: 10.1056/NEJMc0809959.
62. Lapeyraque A., Frémeaux-Bacchi V., Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome // Pediatr. Nephrol. 2011; 26: 621-4. doi: 10.1007/s00467-010-1719-3.
63. Davin J., Gracchi V., Bouts A. et al. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation // Am. J. Kidney Dis. 2010; 55: 708-11. doi: 10.1053/j.ajkd.2009.08.011.
64. FDA approves first therapy for rare inherited form of rickets, x-linked hypophosphatemia (Press release). FDA 17 April 2018.
65. Klinkhoff A. Biological agents for rheumatoid arthritis: targeting both physical function and structural damage // Drugs. 2004; 64: 1267-1283.
66. D’Agati V.D. Renal disease in systemic lupus erythematosus, mixed connective tissue disease, Sjogren’s syndrome, and rheumatoid arthritis / Jennette J.C., Olsen J.L., Schwartz M.M. et al., eds. // Heptinstall’s Pathology of the Kidney. Vol. 1. — Lippincott-Raven: PA, 1998. — 541-624.
67. Hueber A.J., Tunc A., Schett G., Manger B. Anti-tumour necrosis factor alpha therapy in patients with impaired renal function [letter] // Ann. Rheum. Dis. 2007; 66: 981-2. doi: 10.1136/ard.2006.069211.
68. Cho S.K., Sung Y.K., Park S., Bae S.C. Etanercept treatment in rheumatoid arthritis patients with chronic kidney failure on predialysis // Rheumatol. Int. 2010; 30: 1519-22. doi: 10.1007/s00296-009-1108-z.
69. Kobak S. Efficacy and safety of adalimumab in a patient with ankylosing spondylitis on peritoneal dialysis // Rheumatol. Int. 2012; 32: 1785-7. doi: 10.1007/s00296-010-1457-7.
70. Hammoudeh M. Infliximab treatment in a patient with rheumatoid arthritis on haemodialysis // Rheumatology (Oxford). 2006; 45: 357-9. doi: 10.1093/rheumatology/kei264.
71. Joy M.S., Gipson D.S., Powell L., MacHardy J., Jennette J.C., Vento S. et al. Phase 1 trial of adalimumab in focal segmental glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group // Am. J. Kidney Dis. 2010; 55: 50-60. doi: 10.1053/j.ajkd.2009.08.019.
72. Bristol-Myers Squibb Orencia® (abatacept). US Prescri–bing Information. — NJ: Princeton, 2008.
73. Yamada A., Salama A.D., Sayegh M.H. The role of novel T cell costimulatory pathways in autoimmunity and transplantation // J. Am. Soc. Nephrol. 2002; 13: 559-575.
74. Ostor A.J. Abatacept: a T-cell co-stimulation modulator for the treatment of rheumatoid arthritis // Clin. Rheumatol. 2008; 27: 1343-1353. doi: 10.1007/s10067-008-0964-3.
75. Yu C.-C., Fornoni A., Weins A., Hakroush S., Maiguel D., Sageshima J. et al. Abatacept in B7-1-positive proteinuric kidney disease // N. Engl. J. Med. 2013; 369: 2416-23. doi: 10.1056/NEJMoa1304572.
76. Dennler S., Goumans M.J., ten Dijke P. Transforming growth factor beta signal transduction // J. Leukoc. Biol. 2002; 71: 731-740.
77. Yu L., Border W.A., Huang Y., Noble N.A. TGF-beta isoforms in renal fibrogenesis // Kidney Int. 2003; 64: 844-856. doi: 10.1046/j.1523-1755.2003.00162.x.
78. Trachtman H., Fervenza F.C., Gipson D.S., Heering P., Jayne D.R., Peters H. et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-b antibody, in treatment-resistant primary focal segmental glomerulosclerosis // Kidney Int. 2011; 79: 1236-43. doi: 10.1038/ki.2011.33.
79. Vincenti F., Fervenza F., Campbell K. A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis // Kidney Int. Rep. 2017; 2: 800-810. doi.org/10.1016/j.ekir.2017.03.011.
80. Trachtman H., Goyal S., Finn P. et al. Neutralizing –TGF-b in fibrotic renal disorders: focus on fresolimumab // Drugs Future. 2012; 37: 787-794.
81. Dorner T., Shock A., Smith K.G. CD22 and autoimmune disease // Int. Rev. Immunol. 2012; 31: 363-78.

/106-1.jpg)
/107-1.jpg)
/108-1.jpg)
/108-2.jpg)
/108-3.jpg)
/109-2.jpg)
/109-1.jpg)
/110-1.jpg)