Международный эндокринологический журнал Том 15, №3, 2019
Вернуться к номеру
Порівняльна характеристика активності протеолізу за умов експериментального перитоніту та його розвитку на тлі цукрового діабету
Авторы: Grynchuk F.V., Grynchuk A.F.
Bukovinian State Medical University, Chernivtsi, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність зумовлена недостатнім вивченням стану протеолітичних реакцій за поєднання цукрового діабету (ЦД) iз гострим перитонітом (ГП), що дедалі частіше трапляється в практиці. Мета дослідження: вивчення особливостей протеолітичної активності плазми за ГП, що розвивається на тлі ЦД. Матеріали та методи. Сто білих нелінійних щурів. ГП моделювали шляхом черезстравохідної перфорації шлунка, ЦД — уведення 1,6% розчину алоксану. Вивчали протеолітичну активність плазми крові за азоказеїном (АзКз), азоальбуміном (АзАл), азоколагеном (АзКл). Тварини були поділені на групи: інтактні щури, тварини з модельованим ЦД, інтактні щури з модельованим ГП (перша група), тварини з модельованим ГП на тлі ЦД (друга група). Результати. Початковий рівень лізису АзКз і АзАл у тварин з модельованим ЦД був статистично вiрогiдно вищий. Рівень лізису АзКл майже не відрізнявся. Через 6 год з моменту моделювання ГП рівень лізису АзКз збільшився в обох групах, значно більше в першій, але цей показник все одно залишався меншим у першій групі. Значно збільшився рівень лізису АзАл. Рівень лізису АзКл у першій групі майже не змінився, а в другій групі значно зріс. Через 12 год рівень лізису АзКз у першій групі дещо знизився, а в другій групі продовжував зростати. Лізис АзАл суттєво зріс в обох групах, показники в другій групі були вищими. Рівень лізису АзКл у першій групі статистично вiрогiдно знизився, натомість у другій групі значно зріс. Через 24 год рівень лізису АзКз у першій групі суттєво не змінився, а у другій групі продовжував значно зростати. Рівень лізису АзАл у першій групі вірогідно зменшився, натомість у другій групі істотно зріс. Рівень лізису АзКл в обох групах істотно зріс. Через 48 год рівень лізису АзКз і АзАл зріс в обох групах, показники у другій групі були вищими. Рівень лізису АзКл у першій групі дещо знизився, а в другій групі продовжував зростати. Висновки. За експериментального цукрового діабету зростає активність лізису високомолекулярних і низькомолекулярних білків плазми крові. За експериментального гострого перитоніту активується протеолітична система плазми зі збереженням рівноваги між її ланками впродовж 24 годин. Розвиток гострого перитоніту у тварин iз модельованим цукровим діабетом вже через 6 годин суттєво відрізняється за кількісними характеристиками протеолітичної активності плазми крові, що проявляється її надмірним зростанням, розвитком дисбалансу між ланками протеолізу з ознаками виникнення неконтрольованого протеолізу через 24 год. Підґрунтям виявлених відмінностей є зміни функціональної активності протеолітичної системи, зумовлені впливом цукрового діабету, що створює передумови порушень механізмів регуляції запалення.
Актуальность обусловлена недостаточным изучением состояния протеолитических реакций при сочетании сахарного диабета (СД) с острым перитонитом (ОП), которое все чаще встречается в практике. Цель исследования: изучение особенностей протеолитической активности плазмы при ОП, развивающемся на фоне СД. Материалы и методы. Сто белых нелинейных крыс. ОП моделировали посредством чреспищеводной перфорации желудка, СД — введения 1,6% раствора аллоксана. Изучали протеолитическую активность плазмы крови по азоказеину (АзКз), азоальбумину (АзАл), азоколлагену (АзКл). Животные были разделены на группы: интактные крысы, животные с моделированным СД, интактные крысы с моделированным ОП (первая группа), животные с моделированным ОП на фоне СД (вторая группа). Результаты. Исходный уровень лизиса АзКз и АзАл у животных с моделированным СД был статистически достоверно более высоким. Уровень лизиса АзКл почти не отличался. Через 6 ч с момента моделирования ОП уровень лизиса АзКз увеличился в обеих группах, значительно больше в первой, но этот показатель все равно оставался меньшим в первой группе. Значительно увеличился уровень лизиса АзАл. Уровень лизиса АзКл в первой группе почти не изменился, а во второй группе значительно возрос. Через 12 ч уровень лизиса АзКз в первой группе несколько снизился, во второй группе продолжал расти. Лизис АзАл существенно увеличился в обеих группах, показатели во второй группе были выше. Уровень лизиса АзКл в первой группе статистически достоверно снизился, а во второй группе существенно возрос. Через 24 ч уровень лизиса АзКз в первой группе существенно не изменился, а во второй группе продолжал значительно возрастать. Уровень лизиса АзАл в первой группе существенно уменьшился, во второй группе существенно вырос. Уровень лизиса АзКл в обеих группах существенно вырос. Через 48 ч уровень лизиса АзКз и АзАл вырос в обеих группах, показатели во второй группе были выше. Уровень лизиса АзКл в первой группе несколько снизился, а во второй группе продолжал расти. Выводы. При экспериментальном сахарном диабете возрастает активность лизиса высокомолекулярных и низкомолекулярных белков плазмы крови. При экспериментальном остром перитоните активируется протеолитическая система плазмы с сохранением равновесия между ее звеньями в течение 24 часов. Развитие острого перитонита у животных с моделированным сахарным диабетом уже через 6 часов существенно отличается по количественным характеристикам протеолитической активности плазмы крови, что проявляется ее чрезмерным увеличением, развитием дисбаланса между звеньями протеолиза с признаками возникновения неконтролируемого протеолиза через 24 часа. Основой выявленных различий являются изменения функциональной активности протеолитической системы, обусловленные воздействием сахарного диабета, что создает предпосылки нарушений механизмов регуляции воспаления.
Background. The relevance is due to rather understudied state of proteolytic reactions in case of diabetes mellitus (DM) with acute peritonitis (AP), which is increasingly common in the practice. Objective: to study the features of proteolytic activity of plasma in AP associated with DM. Materials and methods. One hundred albino outbred rats. AP was simulated by the transesophageal perforation of the stomach. DM was simulated by the 1.6% alloxan solution injection. The proteolytic activity of blood plasma was studied by azocasein (AzCs), azoalbumin (AzAl), and azocollagen (AzCl). The animals were divided into the following groups: intact rats, animals with simulated DM, intact rats with simulated peritonitis (group 1), animals with models of peritonitis on the background of DM (group 2). Results. The initial level of proteolytic transformation of AzCs and AzAl in animals with simulated DM was significantly higher. The proteolytic transformation level of AzCl had almost no differences. Six hours after the moment AP was modeled, the proteolytic transformation level of AzCs increased in both groups, more significantly in group 1, although this indicator in group 1 remained less. The proteolytic transformation level of AzAl increased significantly. The proteolytic transformation level of AzCl in group 1 remained almost the same, and increased significantly in group 2. In 12 hours, the proteolytic transformation level of AzCs decreased slightly in group 1 but continued to increase in group 2. The proteolytic transformation level of AzAl significantly increased in both groups, and the indicators in group 2 were higher. The proteolytic transformation level of AzCl decreased statistically significantly in group 1, and greatly increased in group 2. In 24 hours, the proteolytic transformation level of AzCs had almost no changes in group 1 but continued to increase in group 2. The proteolytic transformation level of AzAl decreased significantly in group 1 and greatly increased in group 2. The proteolytic transformation level of AzCl significantly increased in both groups. In 48 hours, the proteolytic transformation level of AzCs and AzAl increased in both groups but the indicators in group 2 were higher. The proteolytic transformation level of AzCl decreased slightly in group 1, and continued to increase in group 2. Conclusions. The proteolytic transformation activity of high and low molecular weight blood plasma proteins increases in experimental diabetes mellitus. The proteolytic system of plasma is activated, with maintaining the balance between its links within 24 hours in experimental acute peritonitis. The development of acute peritonitis in animals with simulated diabetes mellitus differs greatly in 6 hours by quantitative characteristics of the proteolytic activity of blood plasma that manifested with its significant increase, some imbalance between the links of proteolysis with the signs of uncontrolled proteolysis in 24 hours. The differences being detected are due to the changes in the functional activity of the proteolytic system caused by diabetes mellitus that underlies the disorders of the mechanisms of inflammation regulation.
цукровий діабет; перитоніт; протеолітична система
сахарный диабет; перитонит; протеолитическая система
diabetes mellitus; peritonitis; proteolytic system
Introduction
The incidence of diabetes mellitus (DM) is constantly growing all over the world in recent years [1–3]. That is why the number of patients with acute peritonitis (AP) which is common [4–6] is growing that is associated with DM. The mechanisms of the development of such comorbid pathology are still unrevealed. In previous reports, the peculiarities of fibrinolytic system with peritonitis on the background of diabetes mellitus were shown [7]. The changes of proteolytic system (РS), the part of which is enzymatic fibrinolysis, still remain unexplored. The role of PS components is crucial in the development of inflammatory process, in particular peritonitis [8, 9]. At the same time, the changes of PS activity are an integral part of DM development mechanisms [10–12]. Therefore, the study of PS reactions in acute peritonitis against the background of diabetes mellitus appears to be rather topical.
The purpose was to study the features of changes in the proteolytic activity of blood plasma in acute peritonitis developing against the background of diabetes mellitus.
Materials and methods
The research has been carried out on 100 albino outbred mature rats weighted 180 to 200 g. The animals were divided into groups: intact rats, animals with simulated DM, intact rats with models of peritonitis (group 1), animals with models of peritonitis on the background of DM (group 2).
Peritonitis was simulated according to the common method by the transesophageal perforation of the sto–mach with the help of a special device [13]. DM was simulated by subcutaneous introduction of 1.6% alloxan solution on distilled water at a dose of 16 mg per 100 g of weight [14].
The main criterion of DM was the blood glucose level within the range of 5.39 ± 0.25 mmol/l (in intact animals — 3.21 ± 0.53 mmol/l, р < 0.01). Peritonitis was induced approximately 3 months after diabetes had been simulated. Blood was taken for analysis before modeling peritonitis, as well as 6, 12, 24, 48 hours after its onset.
While carrying out the study, the researchers adhered to the basic guidelines of Vancouver Convention (1979, 1994) concerning biomedical experiments. The animals were sacrificed by decapitation. All manipulations were performed under the sevorane anesthesia. The Bioethics Committee of HSEI of Ukraine “Bukovinian State Medical University” of the Ministry of Health of Ukraine found the work to be done according to the basic moral and legal principles while conducting the clinical and experimental medical researches.
The proteolytic activity of blood plasma was determined by the level of azocasein (the proteolytic transformation of high molecular weight proteins (HMWP)), azoalbumin (the proteolytic transformation of low molecular weight proteins (LMWP)) and azocollagen (the proteolytic transformation of collagen) according to L.O. Kukharchuk method [15] using Simko Ltd reagents (Lviv, Ukraine).
The hypothesis of normal distribution of data was tested in samples by Shapiro–Wilk test. A verification of the hypothesis of average data equality was carried out by Wilcoxon and Mann–Whitney–Wilcoxon test. The results of the study were statistically processed by the Microsoft® Office Excel (build 11.5612.5703) tables and programs for statistical calculations StatGraphics Plus 5.1 Enterprise edition (2001).
Results
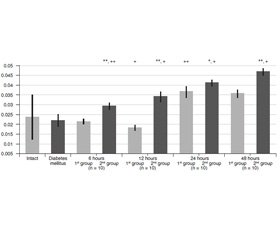
The initial level of proteolytic transformation of azocasein (AzCs) and azoalbumin (AzAl) was significantly higher in animals with simulated DM (Table 1). The proteolytic transformation level of azocollagen (AzCl) had almost no differences.
Six hours after АР was modeled, the proteolytic transformation level of AzCs (Fig. 1) increased in both groups, more significantly in the first one, although this indicator of group 1 remained less. The proteolytic transformation level of AzAl increased significantly (Fig. 2). The proteolytic transformation level of AzCl (Fig. 3) had almost no differences in group 1, and increased greatly in group 2.
In 12 hours, the proteolytic transformation level of AzCs slightly decreased in group 1, and continued to increase in group 2. The proteolytic transformation of AzAl increased significantly in both groups, the indicators of group 2 were significantly higher. The proteolytic transformation level of AzCl decreased statistically significantly in group 1, and increased greatly in group 2.
In 24 hours, the proteolytic transformation level of AzCs didn’t change much in group 1, and continued to increase greatly in group 2. The proteolytic transformation level of AzAl decreased significantly in group 1, increased much in group 2. The proteolytic transformation level of AzCl increased greatly in both groups.
In 48 hours, the proteolytic transformation level of AzCs and AzAl increased in both groups, the indicators of group 2 were higher. The proteolytic transformation level of AzCl decreased slightly in group 1, and conti–nued to increase in group 2.
Discussion
The changes detected in animals with simulated DM indicate an increased level of plasma enzymes. They activate kininogenase, renin as well as angiotensin that is common for DM [9, 16]. A non–contact activation of coagulation factors is an important biological effect of such processes, first of all, factors ХІІ and VІІ as well as a number of other enzymes [17] due to which hypercoagulation develops, which one can face in DM [18]. The object of proteases influence, which modify HMWP, are the components of the complement system. Its activation is noted in DM [19].
Due to kininogenase activation, α2–globulin of the plasma cleaves and then kinins are formed [16, 20]. At the same time, a proteolytic transformation level of AzAl in group 2 indicates the increase of proteases activity. They hydrolyse LMWP, in particular kinins [16]. The activation of proteolytic transformation of kinins may be of regulatory nature. One cannot deny that imbalance between kinin activity dilating the vessels, and angiotensin, which causes vasoconstriction, stimulates the development of circulatory disorders that are common for DM [1, 8]; in addition, the disorders intensify kininase, which converts angiotensin І to angiotensin ІІ [16]. The role of kinins in the realization of the inflammation program is shown [9], their proteolytic transformation is enhanced by one of the factors, which modify the course of inflammation in the peritoneal cavity in AP developing against the background of DM.
Reduced proteolytic transformation level of AzCl in animals with simulated DM indicates the decrease in collagenolysis level. Together with the chronic vasoconstriction as a result of increased formation of angiotensin, it causes vessel wall thickening that is one of the causes of microcirculatory disorders in DM. As α–links of collagen peptides regulate chemotaxis of mononuclear leukocytes, lymphocytes, fibroblasts [21], the decrease of collagen can serve as a precondition of defense systems dysfunction in AP developing against the background of DM.
The increase of proteolytic transformation level of AzCs and AzAl occurs 6 hours after AP simulation in both groups. The increase of proteolytic transformation level of HMWP activates the constriction, kallikrein–kinin, fibrinolytic systems, components of the complement, vasoconstriction, increased vascular permeability, proteases and also influences microorga–nisms–inductors of АР [8, 16]. The increase of proteolytic transformation of LMWP causes cleavage of kinins and biogenic amines [16]. Besides, immunoglobulinase splits immunoglobulin light chains [16], which is definitely the main component of a standard way of the complement activation [19].
At the same time, the proteolytic transformation level of AzCl was fixed in group 1. The collagenolysis activation in group 2 can be interpreted from diffe–rent points of view. On the one hand, this could be due to some need for extra–influence on the vessel walls as their structure changes in DM [1]. On the other hand, increased collagenolysis could be one of the additional factors for activation of cells of the monocyte–macrophage system and lymphocytes taking into conside–ration the chemoattractant and cytoimmunoregulatory properties of the proteolytic transformation of collagen products [19]. In such conditions, a suppression of the synthesizing function of the liver occurs, which is considered to be the main source of protease inhibitors [16], an excessive growth of collagenolysis is the precondition of dysregeneration development, and the destruction of the collagen–like component of C1q complement.
The changes of HMWP proteolytic transformation that were detected in 12 hours can be caused by any reason. In addition, the increase of proteolytic activity is balanced by antiproteolytic factors [16], due to which the level of AzCs proteolytic transformation in group 1 is steady. The changes in liver function caused by DM and toxic affection as a result of AP lead to the inhibition of the synthesis of antiproteolytic protection components [16]. The liver is known to be the main physiological source of proteolytic factors. The increase of HMWP proteolytic transformation in group 2 can be interpreted as a contribution of other donator hydrolases such as activated leukocytes, lymphocytes, endothelial cells, microorganisms, etc. [19].
The high levels of AzAl proteolytic transformation in both groups indicate a sufficient proteases activity. Their effect compensates for some of the negative effects of the outpacing initiation of HMWP proteolytic transformation by regulating the content of biologically active amines. The increase in AzAl proteolytic transformation activity in group 2 was significantly less that indicates a progressive increase in the content of circulating mediators [11, 16, 20, 21].
The decrease of AzCl proteolytic transformation activity in group 1 confirms the activity of collageno–lysis inhibitors. This contributes to the processes of proliferation and delimitation in the site of inflammation [8, 9]. Increased collagenolysis in group 2 leads to a regeneration disorder and is a factor causing the spread of AP.
The changes that were detected in 24 hours indicate the activity of all the proteolytic links in group 2. The activity might become somehow uncontrolled. At the same time, HMWP proteolytic transformation does not increase in the animals of group 1. The level of LMWP even decreases. This indicates the functional activity of proteolysis regulators. However, the increase in the level of collagenolysis suggests some of their dysfunction, which causes the spread of AP.
Other changes detected in 48 hours in group 2 indicate an unlimited activation of proteolysis. The effect of hydrolases and initiators of the proteolytic cascade, which are mutual activators, can be considered as the main cause of this. Destroyed tissues, microorganisms, immunocompetent cells, etc. are considered to be their source in AP [8, 9, 16].
In group 1, the proteolytic transformation level of HMWP and LMWP also increased indicating activation of the cascade of proteolytic reactions. The parameters of AzCl proteolytic transformation didn’t change. Such collagenolysis stability indicates a certain functional activity of proteolysis regulators. Although increased activity of other proteolytic links indicates a lack of these systems.
Conclusions
1. Experimental diabetes mellitus increases the proteolytic transformation activity of high and low molecular weight plasma proteins.
2. In case of experimental acute peritonitis, the proteolytic system of plasma is activated, with maintaining the balance between its links within 24 hours.
3. The development of acute peritonitis in animals with simulated diabetes mellitus in 6 hours significantly differs by its quantitative characteristics of proteolytic activity of blood plasma. This manifested with its excessive growth, the development of an imbalance between proteolysis links with some signs of uncontrolled proteolysis in 24 hours.
4. The basis of such differences are changes in the functional activity of the proteolytic system due to the influence of diabetes mellitus, which creates preconditions for violations of the mechanisms of inflammation regulation.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Information on the contribution of each author: F.V. Grynchuk — the concept and design of the study, analysis of the obtained data; A.F. Grynchuk — the collection and processing of data, text writing.

/197-1.jpg)
/198-1.jpg)
/198-2.jpg)
/198-3.jpg)