Журнал «Медицина неотложных состояний» №6(101), 2019
Вернуться к номеру
Дисфагия в отделении интенсивной терапии: эпидемиология, механизмы и клиническое ведение
Авторы: Мальцева Л.А., Мищенко Е.А., Мосенцев Н.Ф., Мосенцев Н.Н., Голуб А.В.
ГУ «Днепропетровская медицинская академия МЗ Украины», г. Днепр, Украина
Рубрики: Медицина неотложных состояний
Разделы: Справочник специалиста
Версия для печати
Дисфагія може бути в усіх критичних пацієнтів; клінічні дані показують, що постекстубаційна дисфагія (ПЕД) зазвичай спостерігається у пацієнтів відділення інтенсивної терапії (ВІТ). Останні дані показують, що дисфагія в основному зберігається і її наявність незалежно пов’язана з несприятливими клінічними наслідками. Хоча було запропоновано кілька факторів ризику, що, можливо, сприяють розвитку дисфагії, проте точні механізми у пацієнтів ВІТ залишаються в повному обсязі не зрозумілими, і на даний час немає єдиної думки про те, як найкращим чином підходити до пацієнтів ВІТ у групі ризику. З клінічної точки зору, як відомо, дисфагія пов’язана з підвищеним ризиком аспірації та індукованою аспірацією пневмонією, відстроченням відновлення харчування/недоїданням, зниженням якості життя, тривалим перебуванням у ВІТ, а також збільшенням захворюваності та смертності. Крім того, високим є економічне навантаження на систему охорони здоров’я. У світлі високих показників смертності, пов’язаних з дисфагією і спостереженням, що її систематично не перевіряють у більшості ВІТ, цей огляд описує епідеміологію, термінологію і потенційні механізми дисфагії у ВІТ. Крім того, обговорюється вплив дисфагії на окремих людей, систему охорони здоров’я і суспільство у доповнення до сучасних і майбутніх терапевтичних підходів.
Дисфагия может присутствовать у всех критических пациентов; клинические данные показывают, что постэкстубационная дисфагия (ПЭД) обычно наблюдается у пациентов отделения интенсивной терапии (ОИТ). Последние данные свидетельствуют, что дисфагия в основном сохраняется и что ее наличие независимо связано с неблагоприятными клиническими исходами, ориентированными на пациента. Хотя было предложено несколько факторов риска, возможно, способствующих развитию дисфагии, однако лежащие в основе точные механизмы у пациентов в ОИТ остаются не полностью понятными, и в настоящее время нет единого мнения о том, как наилучшим образом подходить к пациентам ОИТ группы риска (ПЭД). С клинической точки зрения дисфагия связана с повышенным риском аспирации и индуцированной аспирацией пневмонии, отсроченным возобновлением питания/недоеданием, снижением качества жизни, длительным пребыванием в ОИТ, а также с увеличением заболеваемости и смертности. Кроме того, экономическая нагрузка на систему общественного здравоохранения является высокой. В свете высоких показателей смертности, связанных с наличием дисфагии и наблюдением, что дисфагию систематически не диагностируют в большинстве ОИТ, этот обзор описывает эпидемиологию, терминологию и потенциальные механизмы дисфагии в ОИТ. Кроме того, обсуждается влияние дисфагии на отдельных пациентов, систему здравоохранения и общество в дополнение к существующим и будущим потенциальным терапевтическим подходам.
Dysphagia may present in all critically ill patients and large-scale clinical data show that e.g. post-extubation dysphagia (PED) is commonly observed in intensive care unit (ICU) patients. Recent data demonstrate that dysphagia is mostly persisting and that its presence is independently associated with adverse patient-centred clinical outcomes. Although several risk factors possibly contributing to dysphagia development were proposed; the underlying exact mechanisms in ICU patients remain incompletely understood and no current consensus exists on how to best approach to ICU patients at risk. From a clinical perspective, dysphagia is well-known to be associated with an increased risk of aspiration and aspiration-induced pneumonia, delayed resumption of oral intake/malnutrition, decreased quality of life, prolonged staying in ICU and hospital, and increased morbidity and mortality. Moreover, the economic burden on public health care systems is high. In light of high mortality rates associated with the presence of dysphagia and the observation that dysphagia is not systematically screened for on most ICUs, this review describes the epidemiology, terminology, and potential mechanisms of dysphagia in the ICU. Furthermore, the impact of dysphagia on affected individuals, health care systems, and society is discussed in addition to current and future potential therapeutic approaches.
розлад ковтання; набута у ВІТ ковтальна дисфункція; набута у ВІТ слабкість; критичні захворювання; сепсис; огляд
расстройство глотания; приобретенная в ОИТ глотательная дисфункция; приобретенная в ОИТ слабость; критические заболевания; сепсис; обзор
deglutition disorder; ICU-acquired swallowing dysfunction; ICU-acquired weakness; critical illness; sepsis; review
Введение
Общая информация
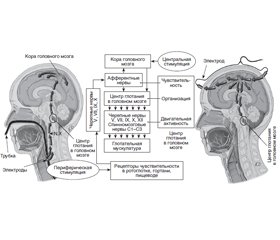
Физиология глотания
Патологическая физиология глотания при критических заболеваниях
Понятие дисфагии в отделении интенсивной терапии
Эпидемиология дисфагии в отделении интенсивной терапии
Факторы риска возникновения дисфагии в отделении интенсивной терапии
Оценка дисфагии у больных в критическом состоянии
Клинические последствия дисфагии у больных в критическом состоянии
Терапевтическая важность.
Общее представление
Изменения в диете и компенсаторные вмешательства
Интервенционный/технологический подход: фарингеальная электростимуляция
Медицинский прогноз
Выводы
1. Brodsky M.B., Gellar J.E., Dinglas V.D., Colantuoni E., et al. Duration of oral endotracheal intubation is associated with dysphagia symptoms in acute lung injury patients. Crit. Care. 2014. 29(4). 574-579.
2. Brown C.V., Hejl K., Mandaville A.D., Chaney P.E., Stevenson G., et al. Swallowing dysfunction after mechanical ventilation in trauma patients. Critical. Care. 2011. 26(1). 108e109-113.
3. Macht M., King C.J., Wimbish T., Clark B.J., et al. Post-extubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Crit. Care. 2013. 17(3). R119.
4. Macht M., Wimbish T., Bodine C., Moss M. ICU-acquired swallowing disorders. Crit. Care Med. 2013. 41(10). 2396-405.
5. Moraes D.P., Sassi F.C., Mangilli L.D., Zilberstein B., et al. Clinical prognostic indicators of dysphagia following prolonged orotracheal intubation in ICU patients. Crit. Care. 2013. 17(5). R243.
6. Zielske J., Bohne S., Brunkhorst F.M., Axer H., et al. Acute and long-term dysphagia in critically ill patients with severe sepsis: results of a prospective controlled observational study. Eur. Arch. Otorhinolaryngol. 2014. 271(11). 3085-93.
7. Skoretz S.A., Flowers H.L., Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010. 137(3). 665-73.
8. Schefold J.C., Berger D., Zurcher P., Lensch M., et al. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. Crit. Care Med. 2017. 45(12). 2061.
9. Brodsky M.B., Huang M., Shanholtz C., Mendez-Tellez P.A., et al. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors. A 5-year longitudinal study. Ann. Am. Thorac. Soc. 2017. 14(3). 376-83.
10. Christensen M., Trapl M. Development of a modified swallowing screening tool to manage post-extubation dysphagia. Nurs. Crit. Care. 2018. 23(2). 102-107.
11. Medeiros G.C., Sassi F.C., Zambom L.S., Andrade C.R. Correlation between the severity of critically ill patients and clinical predictors of bronchial aspiration. Bras. Pneumol. 2016. 42(2). 114-20.
12. Oliveira A.C.M., Friche A.A.L., Salomao M.S., Bougo G.C., et al. Predictive factors for oropharyngeal dysphagia after prolonged orotracheal intubation. Braz. J. Otorhinolaryngol. 2018. 84(6). 722-28.
13. Scheel R., Pisegna J.M., McNally E., Noordzij J.P., et al. Endoscopic assessment of swallowing after prolonged intubation in the ICU setting. Ann. Otol. Rhinol. Laryngol. 2016. 125(1). 43-52.
14. See K.C., Peng S.Y., Phua J., Sum C.L., et al. Nurse-performed screening for postextubation dysphagia: a retrospective cohort study in critically ill medical patients. Crit. Care. 2016. 20(1). 326.
15. Loeb M., McGeer A., McArthur M., Walter S., et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch. Intern. Med. 1999. 159(17). 2058-64.
16. Marik P.E., Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003. 124(1). 328-36.
17. Martin B.J., Corlew M.M., Wood H., Olson D., et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994. 9(1). 1-6.
18. Vergis E.N., Brennen C., Wagener M., Muder R.R. Pneumonia in long-term care: a prospective case-control study of risk factors and impact on survival. Arch. Intern. Med. 2001. 161(19). 2378-81.
19. Ajemian M.S., Nirmul G.B., Anderson M.T., Zirlen D.M., et al. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Archives Surgery (Chicago, Ill, 1960). 2001. 136(4). 434.
20. Barquist E., Brown M., Cohn S., Lundy D., et al. Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: a randomized, prospective trial. Crit. Care Med. 2001. 29(9). 1710-3.
21. Ekberg O., Hamdy S., Woisard V., Wuttge-Hannig A., et al. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002. 17(2). 139-46.
22. El Solh A., Okada M., Bhat A., Pietrantoni C. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003. 29(9). 1451.
23. Holas M.A., DePippo K.L., Reding M.J. Aspiration and relative risk of medical complications following stroke. Arch. Neurol. 1994. 51(10). 1051.
24. Marik P.E. Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 2001. 344(9). 665-71.
25. Schmidt J., Holas M., Halvorson K., Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994. 9(1). 7-11.
26. Tolep K., Getch C.L., Criner G.J. Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest. 1996. 109(1). 167-72.
27. Ponfick M., Linden R., Nowak D.A. Dysphagia — a common, transient symptom in critical illness polyneuropathy: a fiberoptic endoscopic evaluation of swallowing study. Crit. Care Med. 2015. 43(2). 365-72.
28. DeVita M.A, Spierer-Rundback L. Swallowing disorders in patients with prolonged orotracheal intubation or tracheostomy tubes. Crit. Care Med. 1990. 18(12). 1328-30.
29. Daly E., Miles A., Scott S., Gillham M. Finding the red flags: swallowing difficulties after cardiac surgery in patients with prolonged intubation. J. Crit. Care. 2016. 31(1). 119-24.
30. Medeiros G.C., Sassi F.C., Mangilli L.D., Zilberstein B., et al. Clinical dysphagia risk predictors after prolonged orotracheal intubation. Clinics. 2014. 69(1). 8-14.
31. Altman K.W., Yu G.P., Schaefer S.D. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch. Otolaryngol. Head Neck Surg. 2010. 136(8). 784-9.
32. Barker J., Martino R., Reichardt B., Hickey E.J., et al. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can. J. Surg. 2009. 52(2). 119-24.
33. Smithard D.G., O'Neill P.A., Parks C., Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996. 27(7). 1200-4.
34. Brodsky M.B., Gonzalez-Fernandez M., Mendez-Tellez P.A., Shanholtz C., et al. Factors associated with swallowing assessment after oral endotracheal intubation and mechanical ventilation for acute lung injury. Ann. Am. Thorac. Soc. 2014. 11(10). 1545-52.
35. Macht M., White S.D., Moss M. Swallowing dysfunction after critical illness. Chest. 2014. 146(6). 1681-9.
36. Reiter R., Brosch S. Update oropharyngeal dysphagia part 1: physiology, pathology and diagnosis. Laryngorhinootologie. 2012. 91(4). 224-7.
37. Hamdy S., Mikulis D.J., Crawley A., Xue S., et al. Cortical activation during human volitional swallowing: an event-related fMRI study. Am. J. Phys. 1999. 277(1 Pt 1). G219-25.
38. Hamdy S., Rothwell J.C., Brooks D.J., Bailey D., et al. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J. Neurophysiol. 1999. 81(4). 1917-26.
39. Riecker A., Gastl R., Kuhnlein P., Kassubek J., et al. Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia. 2009. 24(1). 114-8.
40. Kumar S. Swallowing and dysphagia in neurological disorders. Rev. Neurol. Dis. 2010. 7(1). 19-27.
41. Lang I.M. Brain stem control of the phases of swallowing. Dysphagia. 2009. 24(3). 333-48.
42. Prosiegel M., Holing R., Heintze M., Wagner-Sonntag E., et al. The localization of central pattern generators for swallowing in humans — a clinical-anatomical study on patients with unilateral paresis of the vagal nerve, Avellis’ syndrome, Wallenberg’s syndrome, posterior fossa tumours and cerebellar hemorrhage. Acta Neurochir. Suppl. 2005. 93. 85-8.
43. Lear C.S., Flanagan J.B., Moorrees C.F. The frequency of deglutition in man. Arch. Oral. Biol. 1965. 10. 83-100.
44. Dodds W.J., Stewart E.T., Logemann J.A. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. Am. J. Roentgenol. 1990. 154(5). 953-63.
45. Reiter R., Brosch S. Update oropharyngeal dysphagia part 2: etiology and therapy. Laryngorhinootologie. 2012. 91(5). 291-9.
46. Stauffer J.L., Olson D.E., Petty T.L. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am. J. Med. 1981. 70(1). 65-76.
47. Suiter D.M., McCullough G.H., Powell P.W. Effects of cuff deflation and one-way tracheostomy speaking valve placement on swallow physiology. Dysphagia. 2003. 18(4). 284-92.
48. Terk A.R., Leder S.B., Burrell M.I. Hyoid bone and laryngeal movement dependent upon presence of a tracheotomy tube. Dysphagia. 2007. 22(2). 89-93.
49. Sue R.D., Susanto I. Long-term complications of artificial airways. Clin. Chest. Med. 2003. 24(3). 457-71.
50. Hong S.J., Lee J.Y. Isolated unilateral paralysis of the hypoglossal nerve after transoral intubation for general anesthesia. Dysphagia. 2009. 24(3). 354-6.
51. Bramer S., Koscielny S., Witte O.W., Terborg C. Bilateral hypoglossal nerve palsy following intubation. Nervenarzt. 2006. 77(2). 204-7.
52. Batjom E., Coron T., Mercier F., Benhamou D. Hypoglossal nerve palsy, a rare complication of orotracheal intubation. Ann. Fr. Anesth. Reanim. 2006. 25(5). 541-2.
53. Dziewas R., Ludemann P. Hypoglossal nerve palsy as complication of oral intubation, bronchoscopy and use of the laryngeal mask airway. Eur. Neurol. 2002. 47(4). 239-43.
54. Schefold J.C., Bierbrauer J., Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J. Cachexia Sarcopenia Muscle. 2010. 1(2). 147-57.
55. Berger D., Bloechlinger S., von Haehling S., Doehner W., et al. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J. Cachexia Sarcopenia Muscle. 2016. 7(4). 403-12.
56. Jolley S.E., Bunnell A.E., Hough C.L. ICU-acquired weakness. Chest. 2016. 150(5). 1129-40.
57. Goldsmith T. Evaluation and treatment of swallowing disorders following endotracheal intubation and tracheostomy. Int. Anesthesiol. Clin. 2000. 38(3). 219-42.
58. Feng X., Todd T., Lintzenich C.R., Ding J., et al. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J. Gerontol. A Biol. Sci Med. Sci. 2013. 68(7). 853-60.
59. Brodsky M.B., De I., Chilukuri K., Huang M., Palmer J.B., et al. Coordination of pharyngeal and laryngeal swallowing events during single liquid swallows after Oral endotracheal intubation for patients with acute respiratory distress syndrome. Dysphagia. 2018. 33(6). 768-77.
60. Aviv J.E. Clinical assessment of pharyngolaryngeal sensitivity. Am. J. Med. 2000. 108(Suppl. 4a). 68S-72S.
61. Aviv J.E., Spitzer J., Cohen M., Ma G., Belafsky P., et al. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002. 112(2). 338-41.
62. Bradley R.M. Sensory receptors of the larynx. Am. J. Med. 2000. 108(Suppl. 4a). 47S-50S.
63. Shaker R., Hogan W.J. Reflex-mediated enhancement of airway protective mechanisms. Am. J. Med. 2000. 108(Suppl. 4a). 8S-14S.
64. Leder S.B., Suiter D.M., Lisitano Warner H. Answering orientation questions and following single-step verbal commands: effect on aspiration status. Dysphagia. 2009. 24(3). 290-5.
65. Boden K., Cedborg A.I., Eriksson L.I., Hedstrom H.W., et al. Swallowing and respiratory pattern in young healthy individuals recorded with high temporal resolution. Neurogastroenterol. Motil. 2009. 21(11).1163-e1101.
66. Perren A., Zurcher P., Schefold J.C. Clinical approaches to assess post-extubation dysphagia (PED) in the critically ill. Dysphagia. 2019. https://doi. org/10.1007/s00455-019-09977-w.
67. Macht M., Wimbish T., Clark B.J., Benson A.B., et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit. Care. 2011. 15(5). R231.
68. Bordon A., Bokhari R., Sperry J., Testa D., et al. Swallowing dysfunction after prolonged intubation: analysis of risk factors in trauma patients. Am. J. Surg. 2011. 202(6). 679-82.
69. Ferraris V.A., Ferraris S.P., Moritz D.M., Welch S. Oropharyngeal dysphagia after cardiac operations. Ann. Thorac. Surg. 2001. 71(6). 1792-5 discussion 1796.
70. Hogue C.W. Jr, Lappas G.D., Creswell L.L., Ferguson T.B. Jr., et al. Swallowing dysfunction after cardiac operations. Associated adverse outcomes and risk factors including intraoperative transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 1995. 110(2). 517-22.
71. Rousou J.A., Tighe D.A., Garb J.L., Krasner H., et al. Risk of dysphagia after transesophageal echocardiography during cardiac operations. Ann. Thorac. Surg. 2000. 69(2). 486-9 discussion 489-490.
72. Skoretz S.A., Yau T.M., Ivanov J., Granton J.T., et al. Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia. 2014. 29(6). 647-54.
73. De Larminat V., Montravers P., Dureuil B., Desmonts J.M. Alteration in swallowing reflex after extubation in intensive care unit patients. Crit. Care Med. 1995. 23(3). 486-90.
74. Leder S.B., Cohn S.M., Moller B.A. Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia. 1998. 13(4). 208-12.
75. Elpern E.H., Scott M.G., Petro L., Ries M.H. Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest. 1994. 105(2). 563-6.
76. Metheny N.A., Clouse R.E., Chang Y.H., Stewart B.J., et al. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit. Care Med. 2006. 34(4). 1007-15.
77. Omura K., Komine A., Yanagigawa M., Chiba N., et al. Frequency and outcome of post-extubation dysphagia using nurse-performed swallowing screening protocol. Nurs. Crit. Care. 2019. 24(2). 70-75.
78. Sassi F.C., Medeiros G.C., Zambon L.S., Zilberstein B., Andrade C.R.F. Evaluation and classification of post-extubation dysphagia in critically ill patients. Rev. Col. Bras. Cir. 2018. 45(3). e1687.
79. Kim M.J., Park Y.H., Park Y.S., Song Y.H. Associations between prolonged intubation and developing post-extubation dysphagia and aspiration pneumonia in non-neurologic critically ill patients. Ann. Rehabil. Med. 2015. 39(5). 763-71.
80. Kwok A.M., Davis J.W., Cagle K.M., Sue L.P., et al. Post-extubation dysphagia in trauma patients: it's hard to swallow. Am. J. Surg. 2013. 206(6). 924-7 discussion 927-928.
81. Doggett D.L., Tappe K.A., Mitchell M.D., Chapell R., et al. Prevention of pneumonia in elderly stroke patients by systematic diagnosis and treatment of dysphagia: an evidence-based comprehensive analysis of the literature. Dysphagia. 2001. 16(4). 279-95.
82. Teuschl Y., Trapl M., Ratajczak P., Matz K., et al. Systematic dysphagia screening and dietary modifications to reduce stroke-associated pneumonia rates in a stroke-unit. PLoS One. 2018. 13(2). e0192142.
83. Marvin S., Thibeault S., Ehlenbach W.J. Post-extubation dysphagia: does timing of evaluation matter? Dysphagia. 2019. 34(2). 210-19.
84. Lynch Y.T., Clark B.J., Macht M., White S.D., et al. The accuracy of the bedside swallowing evaluation for detecting aspiration in survivors of acute respiratory failure J. Crit. Care. 2017. 39. 143.
85. Clave P., Arreola V., Romea M., Medina L., et al. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin. Nutr. 2008. 27(6). 806-15.
86. Antonios N., Carnaby-Mann G., Crary M., Miller L., et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the modified Mann Assessment of Swallowing Ability. J. Stroke Cerebrovasc. Dis. 2010. 19(1). 49-57.
87. Carnaby G.D., Crary M.A. Development and validation of a cancer-specific swallowing assessment tool: MASA-C. Support. Care Cancer. 2014. 22(3). 595-602.
88. Gonzalez-Fernandez M., Sein M.T., Palmer J.B. Clinical experience using the Mann assessment of swallowing ability for identification of patients at risk for aspiration in a mixed-disease population. Am. J. Speech-Lang Pathol. 2011. 20(4). 331-6.
89. Oh J.C., Park J.H., Jung M.Y., Yoo E.Y., et al. Relationship between quantified instrumental swallowing examination and comprehensive clinical swallowing examination. Occup. Ther. Int. 2016. 23(1). 3-10.
90. Hansen T., Lambert H.C., Faber J. Validation of the Danish version of the McGill Ingestive Skills Assessment using classical test theory and the Rasch model. Disabil. Rehabil. 2012. 34(10). 859-68.
91. Lambert H.C., Gisel E.G., Groher M.E., Abrahamowicz M., et al. Psychometric testing of the McGill Ingestive Skills Assessment. Am. J. Occup. Ther. 2006. 60(4). 409-19.
92. Trapl M., Enderle P., Nowotny M., Teuschl Y., et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007. 38(11). 2948-52.
93. Logemann J.A., Veis S., Colangelo L.A. Screening procedure for oropharyngeal dysphagia. Dysphagia. 1999. 14(1). 44-51.
94. Sheppard J.J., Hochman R., Baer C. The dysphagia disorder survey: validation of an assessment for swallowing and feeding function in developmental disability. Res. Dev. Disabil. 2014. 35(5). 929-42.
95. Zhou Z., Salle J., Daviet J., Stuit A., et al. Combined approach in bedside assessment of aspiration risk post stroke: PASS. Eur. J. Phys. Rehabil. Med. 2011. 47(3). 441-6.
96. Maeda K., Shamoto H., Wakabayashi H., Enomoto J., et al. Reliability and validity of a simplified comprehensive assessment tool for feeding support: Kuchi-Kara Taberu Index. J. Am. Geriatr. Soc. 2016. 64(12). e248-52.
97. Lee K.M., Kim H.J. Practical assessment of dysphagia in stroke patients. Ann. Rehabil. Med. 2015. 39(6). 1018-27.
98. Hacki T., Kramer H., Kleinjung C., Perez-Alvarez C., et al. Endoscopic multi-color deglutition study. Laryngorhinootologie. 2001. 80(6). 335-40.
99. Aviv J.E., Kaplan S.T., Thomson J.E., Spitzer J., et al. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): an analysis of 500 consecutive evaluations. Dysphagia. 2000. 15(1). 39-44.
100. Schindler A., Ginocchio D., Peri A., Felisati G., et al. FEESST in the rehabilitation of dysphagia after partial laryngectomy. Ann. Otol. Rhinol. Laryngol. 2010. 119(2). 71-6.
101. Rosenbek J.C., Robbins J.A., Roecker E.B., Coyle J.L., et al. A penetration-aspiration scale. Dysphagia. 1996. 11(2). 93-8.
102. Hannig C., Wuttge-Hannig A., Hess U. Analysis and radiologic staging of the type and severity of aspiration. Radiologe. 1995. 35(10). 741-6.
103. Palmer J.B., Kuhlemeier K.V., Tippett D.C., Lynch C. A protocol for the videofluorographic swallowing study. Dysphagia. 1993. 8(3). 209-14.
104. Fanucci A., Cerro P., Ietto F., Brancaleone C., et al. Physiology of oral swallowing studied by ultrasonography. Dentomaxillofac. Radiol. 1994. 23(4). 221-5.
105. Manabe N., Haruma K., Nakato R., Kusunoki H., et al. New ultrasonographic screening method for oropharyngeal dysphagia: tissue Doppler imaging. Am. J. Physiol. Gastrointest. Liver Physiol. 2018. 314(1). G32-8.
106. Castell J.A., Castell D.O. Modern solid state computerized manometry of the pharyngoesophageal segment. Dysphagia. 1993. 8(3). 270-5.
107. Cook I.J., Dodds W.J., Dantas R.O., Kern M.K., et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989. 4(1). 8-15.
108. Dodds W.J., Hogan W.J., Lydon S.B., Stewart E.T., et al. Quantitation of pharyngeal motor function in normal human subjects. J. Appl. Physiol. 1975. 39(4). 692-6.
109. Dodds W.J., Logemann J.A., Stewart E.T. Radiologic assessment of abnormal oral and pharyngeal phases of swallowing. Am. J. Roentgenol. 1990. 154(5). 965-74.
110. McConnel F.M. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988. 98(1). 71-8.
111. Fattori B., Grosso M., Bongioanni P., Nacci A., et al. Assessment of swallowing by oropharyngoesophageal scintigraphy in patients with amyotrophic lateral sclerosis. Dysphagia. 2006. 21(4). 280-6.
112. Kozlow J.H., Berenholtz S.M., Garrett E., Dorman T., et al. Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit. Care Med. 2003. 31(7). 1930-7.
113. Rassameehiran S., Klomjit S., Mankongpaisarnrung C., Rakvit A. Postextubation dysphagia. Proc. (Bayl. Univ. Med. Cent.). 2015. 28(1). 18-20.
114. Hirst L.J., Sama A., Carding P.N., Wilson J.A. Is a ‘safe swallow’ really safe? Int. J. Lang. Commun. Disord. 1998. 33(Suppl). 279-80.
115. Ohmae Y., Logemann J.A., Kaiser P., Hanson D.G., et al. Effects of two breath-holding maneuvers on oropharyngeal swallow. Ann. Otol. Rhinol. Laryngol. 1996. 105(2). 123-31.
116. Kahrilas P.J., Logemann J.A., Krugler C., Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am. J. Phys. 1991. 260(3 Pt 1). G450-6.
117. Logemann J.A. Treatment of oral and pharyngeal dysphagia. Phys. Med. Rehabil. Clin. N. Am. 2008. 19(4). 803-16 ix.
118. Shanahan T.K., Logemann J.A., Rademaker A.W., Pauloski B.R., et al. Chin-down posture effect on aspiration in dysphagic patients. Arch. Phys. Med. Rehabil. 1993. 74(7). 736-9.
119. Welch M.V., Logemann J.A., Rademaker A.W., Kahrilas P.J. Changes in pharyngeal dimensions effected by chin tuck. Arch. Phys. Med. Rehabil. 1993. 74(2). 178-81.
120. Logemann J.A., Kahrilas P.J., Kobara M., Vakil N.B. The benefit of head rotation on pharyngoesophageal dysphagia. Arch. Phys. Med. Rehabil. 1989. 70(10). 767-71.
121. Prosiegel M., Heintze M., Wagner-Sonntag E., Hannig C., et al. Deglutition disorders in neurological patients. A prospective study of diagnosis, pattern of impairment, therapy and outcome. Nervenarzt. 2002. 73(4). 364-70.
122. Carnaby G., Hankey G.J., Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol. 2006. 5(1). 31-7.
123. Kos M.P., David E.F., Klinkenberg-Knol E.C., Mahieu H.F. Long-term results of external upper esophageal sphincter myotomy for oropharyngeal dysphagia. Dysphagia. 2010. 25(3). 169-76.
124. Shama L., Connor N.P., Ciucci M.R., McCulloch T.M. Surgical treatment of dysphagia. Phys. Med. Rehabil. Clin. N. Am. 2008. 19(4). 817-35 ix.
125. Flint P.W., Purcell L.L., Cummings C.W. Pathophysiology and indications for medialization thyroplasty in patients with dysphagia and aspiration. Otolaryngol. Head Neck Surg. 1997. 116(3). 349-54.
126. Muhle P., Claus I., Marian T., Schroder J.B., et al. Introducing a virtual lesion model of dysphagia resulting from pharyngeal sensory impairment. Neurosignals. 2018. 26(1). 1-10.
127. Teismann I.K., Steinstraeter O., Stoeckigt K., Suntrup S., et al. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007. 8. 62.
128. Hamdy S., Rothwell J.C., Aziz Q., Singh K.D., et al. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat. Neurosci. 1998. 1(1). 64-8.
129. Suntrup S., Marian T., Schroder J.B., Suttrup I., et al. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: a randomized controlled trial. Intensive Care Med. 2015. 41(9). 1629-37.
130. Jayasekeran V., Singh S., Tyrrell P., Michou E., et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010. 138(5). 1737-46.
131. Imoto Y., Kojima A., Osawa Y., Sunaga H., et al. Cough reflex induced by capsaicin inhalation in patients with dysphagia. Acta Otolaryngol. 2011. 131(1). 96-100.
132. Jin Y., Sekizawa K., Fukushima T., Morikawa M., et al. Capsaicin desensitization inhibits swallowing reflex in guinea pigs. Am. J. Respir. Crit. Care Med. 1994. 149(1). 261-3.
133. Nakagawa T., Ohrui T., Sekizawa K., Sasaki H. Sputum substance P in aspiration pneumonia. Lancet. 1995. 345(8962). 1447.
134. Suntrup-Krueger S., Bittner S., Recker S., Meuth S.G., et al. Electrical pharyngeal stimulation increases substance P level in saliva. Neurogastroenterol. Motil. 2016. 28(6). 855-60.
135. Dziewas R., Mistry S., Hamdy S., Minnerup J., et al. Design and implementation of Pharyngeal electrical Stimulation for early de-cannulation in TRACheotomized (PHAST-TRAC) stroke patients with neurogenic dysphagia: a prospective randomized single-blinded interventional study. Int. J. Stroke. 2017. 12(4). 430-7.
136. Dziewas R., Stellato R., Van der Tweel I., Walther E., et al. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): a prospective, single-blinded, randomised trial. Lancet Neurol. 2018. 17(10). 849-59.
137. Marian T., Dunser M., Citerio G., Kokofer A., et al. Are intensive care physicians aware of dysphagia? The MAD (ICU) survey results. Intensive Care Med. 2018. 44(6). 973-5.
138. Van Snippenburg W., Kroner A., Flim M., Hofhuis J., et al. Awareness and management of dysphagia in Dutch intensive care units: a nationwide survey. Dysphagia. 2019. 34(2). 220-28.
139. Dziewas R., Glahn J. Dysphagiemanagement. In: Stefan Schwab, Peter Schellinger, Andreas Unterberger, Christian Werner, Werner Hacker (Hrsg), NeuroIntensiv, 3. Auflage, Springer Verlag, Berlin, Heidelberg. 2015. 108-14.
140. PanEuropean Networks; Science technology (24) 65: firing up the swallowing network, 188-89. Copyright Heike Blum, Department of Neurology, University Hospital Münster, Germany stroke patients with neurogenic dysphagia: a prospective randomized single-blinded interventional study. Int. J. Stroke. 2017. 12(4). 430-7.

/38.jpg)
/39.jpg)
/44.jpg)