Резюме
У дорослих пацієнтів пристрої механічної підтримки кровообігу з безперервним потоком повністю переважають над пульсовими, становлячи понад 90 %. У зв’язку з постійною мініатюризацією приладів все більше дитячих кардіологів пропонують використовувати пристрої механічної підтримки шлуночків iз безперервним потоком у дітей. Відповідно до першого звіту PediMACS, приблизно половина (54 %; 109 з 200) зареєстрованих апаратів для тривалого використання є пристроями з безперервним потоком. У цій статті описано сучасний стан дитячої системи для механічної підтримки кровообігу з безперервним потоком і оцінюються перспективи щодо її використання в майбутньому. Стратегія Сілезького центру хвороб серця в ранньому післяопераційному періоді була дуже схожа з такою, що використовували в пацієнтів після операції Фонтена, включаючи спеціальні тактики респіраторної підтримки, додавання оксиду азоту або залишання грудної клітки відкритою після початку механічної підтримки. Незважаючи на агресивну медичну тактику, у певної частини пацієнтів виникала правошлуночкова недостатність, що потребувало тимчасової механічної підтримки для відновлення кровотоку в малому колі кровообігу і збільшення переднавантаження лівого шлуночка. Рання бівентрикулярна підтримка корелює з кращою виживаністю порівняно з її відкладеним застосуванням. Таким чином, ретельна передопераційна оцінка ризику правошлуночкової недостатності обов’язкова при імплантації пристрою механічної підтримки обох шлуночків або при трансплантації серця.
У взрослых пациентов устройства механической поддержки кровообращения с непрерывным потоком полностью преобладают над пульсовыми, составляя более 90 %. В связи с постоянной миниатюризацией приборов все больше детских кардиологов предлагают использовать устройства механической поддержки желудочков с непрерывным потоком у детей. Согласно первому отчету PediMACS, примерно половина (54 %; 109 из 200) зарегистрированных аппаратов для длительного использования является устройствами с непрерывным потоком. В данной статье описывается современное состояние детской системы для механической поддержки кровообращения с непрерывным потоком и оцениваются перспективы относительно ее использования в будущем. Стратегия Силезского центра болезней сердца в раннем послеоперационном периоде была очень схожа с таковой, применявшейся у пациентов после операции Фонтена, включая специальные тактики респираторной поддержки, добавление оксида азота или оставление грудной клетки открытой после начала механической поддержки. Несмотря на агрессивную медицинскую тактику, у определенной части пациентов возникала правожелудочковая недостаточность, что требовало временной механической поддержки для восстановления кровотока в малом круге кровообращения и увеличения преднагрузки левого желудочка. Ранняя бивентрикулярная поддержка коррелирует с лучшей выживаемостью по сравнению с ее отложенным применением. Таким образом, тщательная предоперационная оценка риска правожелудочковой недостаточности обязательна при имплантации устройства механической поддержки обоих желудочков или при трансплантации сердца.
In adult patients, continuous-flow mechanical circulatory support devices completely prevail over pulsatile ones, representing more than 90 %. With the constant miniaturization of devices, the number of proposals of pediatric cardiologists regarding the use of continuous-flow ventricular assist devices (VAD) in children is growing. According to the first PediMACS report, approximately half (54 %; 109 of 200) of registered long-term devices are continuous-flow devices. This article describes the current state of the children’s continuous-flow system for mechanical circulatory support and evaluates the prospects for its future use. The strategy of the Silesian Center for Heart Diseases in the early postoperative period was very similar to that used in patients after Fontan procedure, including special tactics of respiratory support, adding nitric oxide or leaving the chest open after the start of circulation support. Despite aggressive management, a certain part of the patients had right ventricular failure, which required temporary mechanical support to restore blood flow in the pulmonary circulation and increase left ventricular preload. Early biventricular support correlates with better survival compared to its delayed use. Thus, a thorough preoperative risk assessment of right ventricular failure is required for implantation of biventricular assist device or for heart transplantation.
Introduction
In the last ten years, the mechanical circulatory support (MCS) has become a valuable therapeutic option in patients with end-stage congestive heart failure. Several generations of devices have been developed, but they are mainly designed for adult population. The only available device developed particularly to infants and small children is Berlin Heart pulsatile system. Its superiority in long-term MCS compared to extracorporeal membrane oxygenation (ECMO) has been established [1], demonstrating at the same time many disadvantages, such as neurological complications, cases of pump thrombosis, necessity of readmissions and important limitations of quality of life. At present, the intracorporeal continuous-flow left ventricular assist device (CF-LVAD) provides excellent patient mobility and low complication rates in the adult population. In the adult population, continuous-flow devices completely dominate over pulsatile ones, representing more than 90 % (12,030 of 13,286 primary implants for left heart support) of the durable VADs implanted between 2006 and 2014 [1]. This phenomenon is driven primarily by improved profile of complications and durability of continuous-flow VADs compared with pulsatile ones. With ongoing device miniaturization, enthusiasm has been growing among pediatricians in terms of the use of continuous-flow VADs in children. According to the first PediMACS report, approximately one-half (54 %; 109 of 200) of the long-term devices registered are continuous-flow ones [2]. With the results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing. Continuous-flow VADs compose 62 % (179 of 291) of all durable VAD implants in the PediMACS 2016 third quarter report [3]. In this commentary, we describe the current state of pediatric continuous-flow VAD support, and discuss perspectives regarding its future use.
There is no doubt that continuous-flow VAD technology has a profoundly positive impact on the outcomes of VAD support in the adult population [4]. It is plausible that nearly adult-sized adolescents would have equally excellent outcomes with continuous-flow VADs that were intended for use in adults [5]; however, it is premature to assume that continuous-flow VADs would have a similar impact across the spectrum of body sizes in the pediatric population. Although anecdotal reports with continuous-flow VADs in children are rather encouraging, careful evaluation of available outcomes data is warranted before definitive conclusions can be drawn. Nonetheless, whether smaller children will also experience comparable outcomes with adult-sized continuous-flow VADs remains to be seen. A major concern with the use of continuous-flow VADs in smaller children is a patient-device size mismatch [6], particularly because all currently available continuous-flow VADs are designed for adult patients. A recent multi-institutional study evaluating outcomes after implantation of the HVAD system (Medtronic, Minneapolis) in children with a body surface area (BSA) of < 1.0 m2 showed a favorable survival rate, but with a high incidence of complications, including pump thrombosis [7]. Conway et al. conducted a worldwide survey evaluating the outcome of the HVAD use in children [8]. This study, involving 250 patients from 35 sites in 12 countries, demonstrated a tendency toward poorer outcomes in smaller children; the mortality rate at age < 12 months was 15.6 % (95% confidence interval, 3.96–28.93 %) in children weighing < 25 kg and 7.6 % (95% confidence interval, 6.2–28.9 %) weighing > 25 kg. Although the observed difference in mortality did not reach statistical significance, it warrants close attention from a clinical standpoint. Anecdotally, it is known that both weight and the shape of the chest wall are important factors in successful VAD implantation in children; a smaller child with a broad chest may be a more suitable candidate than a bigger child with a narrow chest. Evaluation by “virtual implantation” such as that used for total artificial heart implantation [9] may provide useful guidance in determining compatibility. Nonetheless, the smaller the heart, the more significant the size mismatch between the patient and the device.
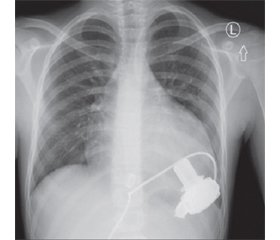
Materials and methods
Between 2016 and 2017, three pediatric patients were implanted with CF-LVAD at the Department of Pediatric Cardiac Surgery, Silesian Center for Heart Diseases (Zabrze, Poland). The indications for initiating MCS were end-stage congestive heart failure due to dilated cardiomyopathy (CMP) in all the patients. Taking into consideration the size of devices, the HeartWare System (HeartWare Inc., Miami Lakes, FL) was chosen for implantation. The system has been previously described in details. Briefly, it consists of a centrifugal pump, an integrated inflow cannula, an outflow graft, and a percutaneous driveline connected to an electronic controller. The pump has the displacement volume of 50 cc and weighs 140 g. The diameter of the pump is 49 mm, the total height is 58 mm. The inflow part has a diameter of 20.5 mm and is 25 mm long. The outflow tube has a diameter of 10 mm, and the percutaneous driveline — a diameter of 4.2 mm. All the implantations were carried out with cardiopulmonary bypass (CPB). A standard median sternotomy was performed during the surgical procedure. The patient was put on cardiopulmonary bypass after sewing the ring to the myocardium and coring the ventricular wall, the inflow cannula was inserted slightly anteriorly to the left ventricular apex. The outflow graft was anastomosed to the ascending aorta using partial clamping. The driveline was then tunneled under the sternum to the right upper abdominal quadrant and connected to the controller. The position of CF-LVAD with respect to the heart could be observed on postoperative chest X-rays of patients. All of them were supported with CF-LVAD alone, despite the fact that they had a high degree right heart dysfunction [10]. In general, the management strategy was similar to that used in patients with Fontan procedure, including nitric oxide application, a special technique of ventilation and even leaving the chest open in the early post-implantation period. A transesophageal and subsequently transthoracic echocardiography were used as a primary tool for monitoring ventricular function and optimization of pump speed while weaning the patient from cardiopulmonary bypass following implantation, and for further adjustments.
Results
Patients’ demographic and preoperative characteristics are listed in Table 1. All the patients met the criteria for INTERMACS score 1 before CF-LVAD implantation. Of three patients forming the study cohort, all received intravenous inotropes prior to CF-LVAD implantation, two were on prolonged mechanical ventilation, while one patient was supported with venous-arterial ECMO. Two patients needed resuscitation procedures to be performed several times.
Clinical outcomes are summarized in Table 2. All the patients remained on VAD support as a bridge-to-transplant. Patient 1 and 2 have been on VAD support over 550 days and continue to be supported. The postoperative chest X-ray images are shown in Fig. 1. None of the patients have developed significant end-organ dysfunction, either in early or long-term follow-up. In none of our patients we have observed symptoms of device malfunction. In patient 1 and 3, signs of depression and feeding intolerance were detected.
/139.jpg)
In all the patients, only LVAD system was implanted in spite of biventricular dysfunction. The prolonged cardiopulmonary bypass time (Table 2) was due to right ventricular dysfunction and attempts to eliminate the implantation of the right ventricular assist device. Postoperative anticoagulation was started with unfractionated heparin (the target activated partial thromboplastin time of 50 to 60 s). As the patients tolerated oral nutrition, heparin was switched to warfarin. The target international normalized ratio (INR) for this cohort was well within the recommended range of 2–3. The platelet inhibitor used was acetylsalicylic acid 1 to 2 mg/kg/day. All the patients and their families were trained prior to discharge home. All the patients received treatment after discharge including metildigoxin, aspirin and acenocoumarol with the dose adjusted according to INR. Patients 2 and 3 additionally received sildenafil, angiotensin-converting enzyme inhibitors and occasionally furosemide. The patients were advised to check INR, initially every 2 days, then on a weekly basis. Readmission occurred two times in patient 1 and one time in patient 2. In patient 1, driveline infection (defined as appearance of erythema or purulent drainage around driveline exit site) was the cause of several readmissions. CF-LVAD-related thrombosis or signs of stroke were not present in any patient. Supraventricular arrhythmia was observed postoperatively in patients 2 and 3, but only in the early post-implantation period. An emergency reference card displaying contact telephone numbers and an algorithm for emergency care was provided to all the patients. Before reintegration to school, school staff members were educated by the family caregiver.
/139_2.jpg)
Discussion
In this study, we have demonstrated that children with low weight and BSA < 1.5 m2 can be successfully implanted with CF-LVAD and supported for long periods. Moreover, all our pediatric patients have been discharged home and attended their schools [6]. There is a growing body of evidence that CF-LVAD is currently the optimal method of long-term MCS, even in pediatric patients. The borderline body weight, when the patients can be supported, is about 20 kg, whereas the pulsatile systems, such as Berlin Heart, remains the option for patients with lower body weight, when the volume of the employed paracorporeal pumps is adjusted to the body mass. One of the major advantages of CF-LVAD systems is the possibility of discharging patients home; they can even attend schools and be active in everyday life. All the patients in our group are physically very active, what may even pose a risk of damaging the driveline. They demonstrated end-stage congestive heart failure, with enhanced pharmacotherapy, being on mechanical ventilation, and in one case on ECMO as a bridge to CF-LVAD. It seems to be one of the causes that the time from admission to discharge home was about one month. With the center gaining experience, the threshold for starting MCS may be optimized, so that the patients might be implanted with CF-LVAD before reaching the critical status, that may influence the post-implantation course. Some data suggest that ECMO should be generally viewed as increasing the risk of LVAD support in the majority of children. On the other hand, it is possible that ECMO [11], when used strictly for short-term resuscitative support to normalize end-organ function immediately prior to VAD implant, may improve LVAD candidacy in selected patients. Patients with significant problems due to left ventricular function very frequently have a concomitant right ventricular dysfunction, so the decision must be reached whether the patient needs LVAD only or biventricular MCS system implantation. The biventricular system is highly demanding and is associated with poorer final results. That is why the majority of centers prefer to start only LVAD and manage patients with right ventricular failure using pharmacological support. Appreciating this strategy, we dedicated much effort to preventing biventricular MCS, which was the cause of prolonged time of cardiopulmonary bypass, which was not related to technical issues of CF-LVAD implantation.
In our experience, the management strategy in the postoperative period was very similar to that in patients after Fontan procedure, including special ventilation settings, nitric oxide administration, or even leaving the chest open shortly after initiation of MCS. Despite aggressive medical support of right ventricle, a certain proportion of patients will still develop right ventricular failure requiring temporary mechanical support to restore blood flow to the pulmonary circulation and increase left ventricular preload [7]. Studies suggest that an early planned biventricular assist device is associated with better survival compared to delayed implantation [8, 9]. Therefore, performing a thorough preoperative risk assessment for right ventricular failure is necessary to implant biventricular assist device or total artificial heart.
Conflicts of interests. Author declares the absence of any conflicts of interests and his own financial interest that might be construed to influence the results or interpretation of his manuscript.
Список литературы
1. Fraser C.D. Jr., Jaquiss R.D., Rosenthal D.N., Humpl T., Canter C.E., Blackstone E.H. et al. Prospective trial of a pediatric ventricular assist device. N. Engl. J. Med. 2012. 367. 532-541.
2. Miera O., Kirk R., Buchholz H., Schmitt K.R., Vander Pluym C., Rebeyka I.M. et al. A multicenter study of the HeartWare ventricular assist device in small children. J. Heart Lung Transplant. 2016. 35. 679-681.
3. Ferro G., Murthy R., Williams D., Sebastian V.A., Forbess J.M., Guleserian K.J. Early outcomes with HeartWare HVAD as bridge to transplant in children: a single institution experience. Artif. Organs. 2016. 40. 85-89.
4. Padalino M.A., Bottio T., Tarzia V., Bortolussi G., Cerutti A., Vida V.L. et al. HeartWare ventricular assist device as bridge to transplant in children and adolescents. Artif. Organs. 2014. 38. 418-422.
5. Peng E., Kirk R., Wrightson N., Duong P., Ferguson L., Griselli M. et al. An extended role of continuous flow device in pediatric mechanical circulatory support. Ann. Thorac. Surg. 2016. 102. 620-627.
6. Schweiger M., Vanderpluym C., Jeewa A. et al. Outpatient management of intra-corporeal left ventricular assist device system in children: a multi-center experience. American Journal of Transplantation. 2015. 15. 453-460.
7. Haneya A., Philipp A., Puehler T. et al. Temporary percutaneous right ventricular support using a centrifugal pump in patients with postoperative acute refractory right ventricular failure after left ventricular assist device implantation. Eur. J. Cardiothorac. Surg. 2012. 41. 219-223.
8. Fitzpatrick J.R. 3rd, Frederick J.R., Hiesinger W. et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J. Thorac. Cardiovasc. Surg. 2009. 137. 971-977.
9. Morgan J.A., John R., Lee B.J. et al. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann. Thoracic Surg. 2004. 77. 859-863.
10. Cheung A.W., White C.W., Davis M.K., Freed D.H. Short-term mechanical circulatory support for recovery from acute right ventricular failure: Clinical outcomes. J. Heart Lung Transplant. 2014. 33. 794-799.
11. Riebandt J., Haberl Th., Wiedemann D. et al. Extracorporeal membrane oxygenation support for right ventricular failure after left ventricular assist device implantation. Eur. J. Cardiothorac. Surg. 2018. 53. 590-595.

/138.jpg)
/139.jpg)
/139_2.jpg)