Журнал «Практическая онкология» Том 2, №3, 2019
Вернуться к номеру
Иммунозависимые побочные эффекты, ассоциированные с иммунотерапией рака
Авторы: Головач И.Ю. (1), Егудина Е.Д. (2)
1 - Клиническая больница «Феофания» Государственного управления делами, г. Киев, Украина
2 - Клиника современной ревматологии, г. Киев, Украина
Рубрики: Онкология
Разделы: Справочник специалиста
Версия для печати
Імунотерапія ознаменувала настання нової ери в онкології. Інгібітори імунних контрольних точок (іІКТ) підсилюють протипухлинний імунітет, активізуючи імунну систему пацієнта для боротьби з раком. Показано, що ці агенти мають більш високий профіль безпеки, ніж хіміотерапія. Незважаючи на те, що терапія цим класом препаратів призвела до поліпшення клінічних результатів у пацієнтів із множинними типами пухлин, на тлі терапії іІКТ розвивається широкий спектр імунозалежних побічних ефектів (ІЗПЕ), що зачіпають будь-яку систему органів, з різними клінічними проявами. Більшість токсичних ефектів є зворотними, але оперативне розпізнавання і лікування ІЗПЕ асоційовані з поліпшенням результатів терапії іІКТ і являють собою важливе нове клінічне завдання для практикуючих онкологів. В даному літературному огляді наведені найбільш поширені і клінічно важливі ІЗПЕ з описом їх епідеміології, клінічних проявів і лікування. Оскільки імунотерапія на сьогодні все ширше використовується в клінічній практиці, вкрай важливо міждисциплінарне співробітництво онкологів з ревматологами, пульмонологами, ендокринологами, гематологами, нефрологами, окулістами і дерматологами для ранньої діагностики і оптимального менеджменту. Також розглянуті майбутні стратегії, що можуть дати змістовне уявлення про профілактику і менеджмент ІЗПЕ.
Иммунотерапия ознаменовала наступление новой эры в онкологии. Ингибиторы иммунных контрольных точек (иИКТ) усиливают противоопухолевый иммунитет, активизируя иммунную систему пациента для борьбы с раком. Показано, что эти агенты имеют более высокий профиль безопасности, чем химиотерапия. Несмотря на то что терапия этим классом препаратов привела к улучшению клинических результатов у пациентов с множественными типами опухолей, на фоне терапии иИКТ развивается широкий спектр иммунозависимых побочных эффектов (ИЗПЭ), затрагивающих любую систему органов, с различными клиническими проявлениями. Большинство токсических эффектов являются обратимыми, но оперативное распознавание и лечение ИЗПЭ ассоциированы с улучшением результатов терапии иИКТ и представляют собой важную новую клиническую задачу для практикующих онкологов. В данном литературном обзоре представлены наиболее распространенные и клинически важные ИЗПЭ с описанием эпидемиологии, клинических проявлений и лечения. Поскольку иммунотерапия в настоящее время все шире используется в клинической практике, крайне важно междисциплинарное сотрудничество онкологов с ревматологами, пульмонологами, эндокринологами, гематологами, нефрологами, окулистами и дерматологами для ранней диагностики и оптимального менеджмента. Также рассмотрены будущие стратегии, которые могут дать содержательное представление о профилактике и менеджменте ИЗПЭ.
Immunotherapy marked the onset of a new era in oncology. Immune checkpoint inhibitors enhance anti-tumor immunity by activating the patient’s immune system to fight cancer. It has been shown that these agents have a higher safety profile than chemotherapy. And despite the fact that therapy with this class of drugs has led to an improvement of clinical results in patients with multiple types of tumors, a wide range of immune-related adverse events with various clinical manifestations affecting any organ system develops on the background of immune checkpoint inhibitor therapy. Most of the toxic effects are reversible, but the prompt recognition and treatment of immune-related adverse events is associated with improved outcomes of immune checkpoint inhibitor therapy and represents an important new clinical challenge for practicing oncologists. This literature review presents the most common and clinically important immune-related adverse events, with epidemiology, clinical presentation, and treatment. As immunotherapy is currently being increasingly used in clinical practice, the interdisciplinary cooperation of oncologists with rheumatologists, pulmonologists, endocrinologists, hematologists, nephrologists, ophthalmologists and dermatologists is extremely important for early diagnosis and optimal management. Future strategies that can provide a meaningful insight into the prevention and management of immune-related adverse events are also considered.
інгібітори імунних контрольних точок; імунозалежні побічні ефекти; імунотерапія; імунні токсичні ефекти; менеджмент
ингибиторы иммунных контрольных точек; иммунозависимые побочные эффекты; иммунотерапия; иммунные токсические эффекты; менеджмент
immune checkpoint inhibitors; immune-related adverse events; immunotherapy; immune-related toxicities; management
Уже более 50 лет хирургия, лучевая терапия и химио–терапия являются основным оружием врача–онколога в борьбе со злокачественными новообразованиями. С развитием иммунотерапии наметился существенный прорыв в лечении пациентов со злокачественными новообразованиями [1]. Ускользание от распознавания иммунной системой и устойчивость к ее воздействию являются одними из основных признаков раковых клеток [2].
В микроокружении опухоли отмечается сверхэкспрессия иммуносупрессивных молекул, таких как цитотоксический T–лимфоцит–ассоциированный антиген 4 (CTLA–4), белок программируемой клеточной гибели (PD–1) и его лиганд PD–L1 [2]. Это так называемые иммунные контрольные точки (ИКТ), которые являются регуляторами иммунной активации и иммунного ответа. Опухолевые клетки используют эти контрольные точки для предотвращения активации опухоль–специфических лимфоцитов, тем самым препятствуя воздействию клеток иммунного надзора, что способствует неконтролируемому росту опухоли. Кроме того, ИКТ контролируют и предотвращают запуск аутоиммунных процессов, модулируют их, уменьшая вызванные иммунными клетками повреждения в органах и тканях. Нацеливаясь на молекулы CTLA–4 и –PD–1, блокаторы (ингибиторы) иммунных контрольных точек (иИКТ) реактивируют цитотоксические Т–клетки, которые разрушают опухолевые клетки и ликвидируют этот ключевой механизм прогрессирования рака, активируя иммунную систему для уничтожения раковых клеток [2].
Первыми ингибиторами контрольных точек иммунитета с высокой противоопухолевой активностью были моноклональное анти–CTLA–4–антитело ипилимумаб [3] и анти–PD–1–антитела ниволумаб и пембролизумаб, которые первоначально продемонстрировали высокую активность при меланоме [4], а затем и при других формах рака.
Ингибиторы ИКТ продемонстрировали беспрецедентную частоту ответа на широкий спектр форм рака, в настоящее время семь иИКТ одобрены Управлением по санитарному надзору за качеством пищевых продуктов и медикаментов (FDA) и имеют более чем 14 различных показаний к лечению рака (табл. 1).
Активация иммунной системы на фоне использования иИКТ может привести к уникальному спектру токсичности, известному как иммунозависимые побочные эффекты (ИЗПЭ). В настоящее время специалисты, используя иИКТ в клинической практике, все чаще сталкиваются с диагностической и терапевтической проблемой идентификации ИЗПЭ и ведения пациентов с этими симптомами.
Высокий риск развития ИЗПЭ при использовании иИКТ требует полноценного участия в лечении и исследовании таких пациентов мультидисциплинарной команды врачей–специалистов разного профиля наряду с онкологами. Это связано с тем, что данные проявления могут быть опасными для жизни, проявляться поражением различных органов и систем и иногда трансформироваться в хронические заболевания, существенно снижающие качество жизни и доминирующие в клинической картине.
В поздних фазах клинических исследований было отмечено, что распространенность ИЗПЭ колеблется от 15 до 90 % при иИКТ [5]. При этом фульминантные ИЗПЭ, приводящие к смерти пациентов, регистрируются с частотой 0,3–1,3 % [6]. Риск, клинические проявления и тяжесть ИЗПЭ варьируют в зависимости от режима терапии иИКТ и типа рака. Частота и степень тяжести ИЗПЭ наиболее высоки среди пациентов, получающих комбинированную терапию ипилимумаб/ниволумаб по сравнению с монотерапией [7]. При назначении терапии анти–CTLA–4 чаще имеют место такие иммуноопосредованные побочные эффекты, как колит и гипофизит, тогда как у пациентов, получающих терапию анти–PD–1, чаще встречаются пневмонит и тиреоидит [8, 9].
Тяжесть неблагоприятных событий и токсичность режимов можно классифицировать, используя общие терминологические критерии для нежелательных явлений Национального института рака США (Common terminology criteria for adverse events grading — CTCAE) (табл. 2) [10].
Хотя эта оценочная система используется в онкологии в течение многих лет, целенаправленно она не предназначена для ведения пациентов с ИЗПЭ. В ответ на этот клинический вызов в отношении распознавания данного класса токсичности и ведения таких пациентов ведущими онкологическими обществами, в том числе Американским обществом клинической онкологии (ASCO), Европейским обществом медицинской онкологии (ESMO), рабочей группой, изучающей онкологическую токсичность иммунотерапии и Национальной комплексной онкологической сетью США (NCNN), в 2017–2018 гг. были опубликованы всеобъемлющие руководящие принципы для улучшения соответствующей оценки и лечения ИЗПЭ [10–12].
Эти рекомендации в значительной степени основаны на ретроспективных данных и мнении экспертов. Учитывая отсутствие данных проспективных клинических исследований, посвященных редким и молниеносным клиническим проявлениям ИЗПЭ, данные состояния остаются особенно сложными в диагностике и менеджменте для врачей. В то время как лечение варьирует в зависимости от тяжести ИЗПЭ, терапия иИКТ может быть продолжена для большинства пациентов с 1–й степенью токсичности и прекращена для пациентов со 2–й степенью и выше. Исходя из рекомендаций терапию следует возобновить после разрешения симптомов токсичности 2–й или 3–й степени, однако при ИЗПЭ 4–й степени терапию следует прекратить полностью [13]. При этом безопасность и польза повторного назначения иИКТ неизвестны, решение о возобновлении лечения следует рассматривать в каждом конкретном случае отдельно. В недавнем исследовании пациентов с немелкоклеточным раком легких (НМРЛ) повторное назначение анти–PD–L1 терапии привело к рецидивам ИЗПЭ у 52 % пациентов [14].
Широко признано, что эффективный менеджмент ИЗПЭ зависит от раннего распознавания и своевременного вмешательства [13]. Клиническая настороженность, своевременная оценка и междисциплинарное ведение пациентов обеспечивают основу для оптимальных клинических исходов лечения больных иИКТ (рис. 1).
Хотя любая система органов может быть поражена вследствие ИЗПЭ, чаще всего в патологический процесс вовлекаются желудочно–кишечный тракт, эндокринные железы, кожа и печень [15], более редкими являются поражения костно–мышечной, центральной нервной (ЦНС) и сердечно–сосудистой систем, легких и органов кроветворения. Клинический спектр ИЗПЭ представлен на рис. 2.
Поражение кожи
Дерматологическая токсичность является наиболее часто встречающимся ИЗПЭ и наблюдается у 30–50 % пациентов, получающих терапию иИКТ [16], из них 37–70 % пациентов получают анти–CTLA–4 терапию и 17–37 % — анти–PD–1/PD–L1 терапию. Из них менее чем у 3 % развивается токсичность выше 3–й степени тяжести. Дерматологические ИЗПЭ имеют различные клинические проявления и сроки начала. Клинические симптомы варьируют от зуда, витилиго, воспалительных высыпаний (реакции кожной гиперчувствительности, макулопапулезная, угревая, эксфолиативная и псориазообразная форма сыпи), буллезных дерматозов (буллезный пемфигоид, буллезная лекарственная реакция) до тяжелых и жизнеугрожающих (синдром Стивенса — Джонсона, токсический эпидермальный некролиз, синдром лекарственной гиперчувствительности с эозинофилией и системными проявлениями). Время возникновения может колебаться от 2 недель до нескольких месяцев от начала терапии иИКТ [17].
Однако следует отметить, что развитие ИЗПЭ в виде поражения кожи коррелирует с лучшим клиническим ответом и большей выживаемостью у пациентов с метастатической меланомой на фоне терапии пембролизумабом или ниволумабом [18, 19]. Развитие витилиго на фоне лечения иИКТ также продемонстрировало значительную связь как с лучшей выживаемостью без прогрессирования опухоли, так и с общей выживаемостью в метаанализе 27 исследований пациентов с меланомой, получавших широкий спектр иммунотерапевтических препаратов [20].
Диагностическая оценка. У пациентов с дерматологическими ИЗПЭ необходимо собрать подробный клинический анамнез и провести физикальное обследование с тщательной оценкой всей поверхности кожных покровов, слизистых оболочек и лимфатических узлов с особым акцентом на процент площади пораженной кожи и наличие или отсутствие пузырей. Положительный признак Никольского (индукция образования пузырей посредством механического давления) должен настораживать врача относительно развития синдрома Стивенса — Джонсона, при котором поражаются слизистые оболочки и наблюдаются системные проявления (лихорадка, слабость, сонливость, конституциональные симптомы). При биопсии кожи в > 90 % случаев для таких пациентов типично наличие линейного отложения иммуноглобулина G (IgG) и линейное окрашивание С3 вдоль базальной мембраны [21]. Если биопсия невозможна, сыворотку можно отправить на определение уровня антител к BP180 и BP230 (тест ELISA) для подтверждения диагноза пузырного дерматоза — пемфигоида [21].
Менеджмент пациентов. За исключением буллезного поражения, большинство проявлений кожной токсичности 1–й и 2–й степени можно лечить с помощью местной терапии (смягчающие средства, мази с глюкокортикоидами) с продолжением терапии иИКТ. Пациент в обязательном порядке должен быть проконсультирован дерматологом. Эскалация терапии включает прекращение иммунотерапии, более частое использование топических глюкокортикоидов (ГК) и назначение системных ГК [13]. При 3–4–й степени токсичности следует назначать внутривенный (в/в) метилпреднизолон (МП) в дозе 1–2 мг/кг в течение 3–5 дней с переходом на оральные формы из расчета 0,5–1 мг/кг с постепенным снижением дозы через 3–4 недели по мере разрешения кожных проявлений [11]. Пациенты с > 30% поражением поверхности тела должны находиться в специализированном дерматологическом отделении. В стероидно–резистентных случаях альтернативным подходом считается применение в/в иммуноглобулина, ритуксимаба или циклоспорина в сочетании с ГК и локальной терапией [13]. Примечательно, что кожные ИЗПЭ были признаны в качестве основного барьера для приверженности пациента к терапии иИКТ [10].
Поражение желудочно–кишечного тракта
Наиболее частыми проявлениями токсичности со стороны желудочно–кишечного тракта (ЖКТ) в результате применения анти–CTLA–4 являются диарея и колит. Частота возникновения колита составляет 8–27 %, диареи — ~ 54 %, причем 37 % смертельных исходов на фоне лечения иИКТ ассоциированы именно с поражением ЖКТ [6]. Чаще гастроинтестинальные ИЗПЭ наблюдаются у пациентов, получавших монотерапию анти–CTLA–4 по сравнению с комбинированной терапией анти–CTLA–4/PD–1 [22, 23]. При непосредственном сравнении ингибиторов PD–1 с ингибиторами CTLA–4 относительный риск развития диареи и колита составил 0,58 и 0,16 соответственно [24]. Время начала гастроинтестинальных ИЗПЭ обычно составляет 5–10 недель после начала терапии иИКТ [25].
Описана также иИКТ–индуцированная гепатотоксичность с развитием аутоиммунного гепатита, частота которого составляет 2–10 % у пациентов, получающих монотерапию, и 25–30 % у пациентов, получающих комбинированную терапию PD–1/CTLA–4 [26]. Гепатит является вторым наиболее распространенным ИЗПЭ, приводящим к летальному исходу при анти–PD–1/PD–L1 терапии [6]. Время развития гепатита, как ИЗПЭ, составляет 6–12 недель после начала лечения [27]. Другие, более редкие гастроинтестинальные ИЗПЭ включают дисфагию, гастрит, дуоденит и панкреатит [28].
Диагностическая оценка. Симптомы поражения кишечника на фоне терапии иИКТ включают боль в животе, чаще спастического характера, тошноту, вздутие, кровь или слизь в кале и собственно нарушение стула. Диагностическое обследование у пациентов с подозрением на наличие иИКТ–индуцированного колита включает стандартное лабораторное тестирование для исключения инфекционной и неинфекционной этиологии, включая общий анализ крови (ОАК), биохимическое исследование крови, бактериологический посев кала, определение С–реактивного белка (СРБ), кальпротектина и/или лактоферрина для мониторинга активности заболевания, ответа на лечение и дифференциации с синдромом раздраженного кишечника [11]. У пациентов с тяжелым колитом необходимо провести исследования для исключения туберкулезной инфекции, ВИЧ, вирусных гепатитов с целью подготовки к возможной терапии ингибиторами фактора некроза опухоли (ФНО–a) при стероид–резистентном колите [11]. На компьютерной томографии (КТ) брюшной полости с контрастированием для иИКТ–индуцированного колита характерен застой в брыжеечных сосудах, утолщение стенки толстой кишки, растяжение кишечника и уплотнение периколитической клетчатки [27]. При затруднении диагностики и/или для оценки тяжести колита следует провести прямую визуализацию толстой кишки с помощью колоноскопии или гибкой сигмоидоскопии. Наличие изъязвления при прямой визуализации, как правило, ассоциировано со стероид–рефрактерным течением колита, и в этих случаях необходимо как можно более раннее назначение ингибиторов ФНО–a (инфликсимаба или адалимумаба). Сообщалось, что иИКТ–индуцированный колит преимущественно поражает нисходящую ободочную кишку [27].
Менеджмент. Пациентам с колитом 1–2–й степени токсичности следует воздержаться от терапии иИКТ, лечение следует возобновлять только в случае разрешения гастроинтестинальных проявлений. При развитии токсичности 3–4–й степени рекомендуется прекращение терапии анти–CTLA–4 [25]. Однако указывается, что терапию анти–PD–1, PD–L1 можно возобновить при восстановлении состояния кишечника. Необходимо назначение ГК в дозе < 10 мг/кг/сут (в преднизоновом эквиваленте) при 2–й степени токсичности, 1–2 мг/кг/сут — при 3–4–й с постепенным уменьшением дозы в течение 4–6 недель при достижении 1–й степени токсичности [11]. Следует проводить поддерживающую терапию при помощи регидратации, электролитных растворов и антидиарейных агентов. При отсутствии адекватного клинического ответа в течение 48–72 ч после терапии ГК следует начать терапию ингибитором ФНО–a инфликсимабом 5–10 мг/кг с повторной инъекцией через 2 недели [12]. Ведолизу–маб — анти–интегриновое антитело a4b7 — следует рассматривать у пациентов, у которых развивается колит, резистентный к инфликсимабу, или в случаях, когда анти–ФНО–a терапия противопоказана (сопутствующая тяжелая инфекция, демиелинизирующие заболевания и тяжелая сердечная недостаточность) [13]. Повторная колоноскопия не является обязательной и может быть предложена для случаев 2–й степени токсичности или выше для мониторинга активности заболевания, для оценки достижения полной ремиссии, особенно при планировании возобновления терапии иИКТ.
Поражение гепатобилиарной системы
ИИКТ–ассоциированный гепатит проявляется необъяснимым повышением сывороточных уровней печеночных ферментов — аланинаминотрансферазы (АлАТ) или аспартатаминотрансферазы (АсАТ). Распространенность этого побочного эффекта колеблется в диапазоне 2–10 % [30]. Однако комбинированное лечение ипилимумабом и ниволумабом увеличивает частоту гепатитов до 25–30 %, при этом токсичность 3–й степени регистрируется в 15 % случаев [31]. Симптомы развиваются преимущественно в течение первых 6–12 недель от старта лечения. Большинство иИКТ–ассоциированных гепатитов протекают бессимптомно и проявляются только изменениями уровней печеночных ферментов [30].
Диагностическая оценка. При ведении таких пациентов в обязательном порядке необходимо исключить вирусную инфекцию, а именно гепатит А, В, С и Е. КТ или ультразвуковое исследование (УЗИ) печени и желчевыводящих путей позволяют исключить метастатический процесс или желчнокаменную болезнь. У некоторых пациентов с иИКТ–ассоциированным гепатитом может отмечаться незначительная гепатомегалия, перипортальный отек или лимфаденопатия [30]. Аутоиммунный гепатит на фоне лечения иИКТ характеризуется отрицательными тестами на антинуклеарные антитела (ANA), антитела к гладкой мускулатуре, антитела к микросомам печени и почек [32]. Достоверный диагноз аутоиммунного гепатита требует биопсии печени с обнаружением диффузной инфильтрации Т–клетками, выраженными синусоидальными гистиоцитарными инфильтратами и повреждением центральной вены [32].
Менеджмент. Пациентам необходимо назначить ГК [32] в дозе, аналогичной для лечения колита, а в стероид–резистентных случаях (при отсутствии улучшения в течение 3 дней) к лечению следует добавлять азатиоприн или микофенолата мофетил в соответствии с рекомендациями по лечению аутоиммунного гепатита [33]. Инфликсимаб не является подходящим вариантом лечения в ситуации иммуноопосредованного гепатита, в отличие от колитов, учитывая потенциальный риск идиосинкратической печеночной недостаточности.
Поражение эндокринной системы
Поражения эндокринной системы в виде ИЗПЭ представляют значимую клиническую проблему, симптомы которой часто не распознаются врачами. Жалобы таких пациентов являются неспецифичными и включают тошноту, рвоту, головокружение, головную боль, изменение зрения, аритмии, повышенную потливость, необъяснимые колебания массы тела, чувство голода или жажды больше, чем обычно, выпадение волос, изменения настроения или поведения, усталость, вялость и общее недомогание. Такой полиморфизм жалоб значительно затрудняет диагностику. Чаще всего поражаются гипофиз, щитовидная и поджелудочная железы и надпочечники, хотя также имеется ряд наблюдений о поражении паращитовидных желез [34]. Частота эндокринопатий, связанных с лечением иИКТ, составляет приблизительно 10 % в недавнем метаанализе 7551 пациента, получавших иИКТ [35]. Риск эндокринных ИЗПЭ существенно возрастает при комбинированной терапии, чаще всего это гипотиреоз (17 %), гипофизит (13 %) и гипертиреоз (10 %) [36].
Гипофизит
Диагностическая оценка. У пациентов с подозрением на гипофизит следует обследовать систему «гипоталамус — гипофиз», включая определение свободного тироксина (Т4), тиреотропного гормона (ТТГ), лютеинизирующего гормона (ЛГ), фолликулостимулирующего гормона (ФСГ), адренокортикотропного гормона (АКТГ) и кортизола, а также сывороточные электролиты. Для последующей тактики лечения крайне важно различать первичный и вторичный гормональный дефицит. Клиницисты должны понимать, что гипофизит может привести к вторичной надпочечниковой недостаточности и гипотиреозу. Неспособность распознать это заболевание имеет негативные последствия для ведения пациента. Магнитно–резонансная томография (МРТ) головного мозга с контрастированием должна быть назначена пациентам с эндокринными нарушениями, с впервые выявленными сильными головными болями и при нарушении зрения.
Менеджмент. При развитии гипофизита рекомендуется воздержаться от любого вида терапии иИКТ. При стабилизации состояния на фоне заместительной гормональной терапии лечение иИКТ может быть во–зобновлено. При развитии гипофизита высокого уровня токсичности (3 и выше) пациентам необходимо назначить начальную пульс–терапию ГК с последующим назначением высоких дох per os [12]. Необходимо контролировать уровень свободного Т4, при дефиците которого следует назначать левотироксин [13]. Ключевые понятия менеджмента включают высокий индекс клинических подозрений со стороны врача, определение локализации эндокринной дисфункции, назначение заместительной терапии гормонами и тщательный мониторинг. Ассоциированные с применением иИКТ поражения эндокринной системы являются жизне–угрожающими, поскольку они часто необратимы и пациенты требуют пожизненной заместительной гормональной терапии [37].
Нарушение функции щитовидной железы (гипотиреоз, гипертиреоз)
Гипотиреоз является одним из наиболее распространенных эндокринных ИЗПЭ на фоне лечения иИКТ. Систематический обзор и метаанализ R. Barroso–Sousa и соавт. (2018) продемонстрировал, что общая частота гипотиреоза на фоне иИКТ составила 6,6 % [36]. Гипотиреоз может проявляться усталостью, непреднамеренным увеличением массы тела, непереносимостью холода, запорами, миалгией и сухостью кожи. Гипертиреоз, наоборот, является достаточно редким проявлением, его частота составляет 0,3–1 % [36]. Пациенты, как правило, жалуются на перебои в работе сердца, тахикардию, повышенную потливость, необъяснимую потерю массы тела, раздражительность, возбудимость, нарушение сна.
Диагностическая оценка. При физикальном обследовании при подозрении на гипотиреоз можно обнаружить зоб, брадикардию, диастолическую гипертензию, снижение сухожильных рефлексов. ТТГ и свободный T4 должны быть проверены до начала терапии иИКТ, в дальнейшем их контроль должен осуществляться каждые 4–6 недель или по мере необходимости для симптомных пациентов [38]. Важно отличать первичный гипотиреоз от вторичного, а также дифференцировать гипотиреоз от поздней фазы тиреоидита. Повышенный уровень ТТГ с низким значением свободного T4 свидетельствует о биохимическом гипотиреозе, при обнаружении которого следует также исследовать уровень антител к пероксидазе щитовидной железы [13]. При клинических признаках, вызывающих подозрение на возникновение болезни Грейвса (офтальмопатия, аритмия, неврологические симптомы), следует проверить наличие антител к рецептору ТТГ.
Менеджмент. У пациентов с гипотиреозом и гипертиреозом 1–й степени токсичности иИКТ могут быть продолжены на фоне тщательного мониторинга ТТГ и свободного Т4. Для токсичности 2–й степени и гипотиреозе следует назначать соответствующую заместительную гормональную терапию либо с продолжением терапии ИИКТ, либо с временной отменой до тех пор, пока у пациентов сохраняются симптомы с любым уровнем повышения ТТГ или у пациентов с бессимптомным течением и уровнями ТТГ > 10 мМЕ/л [39]. Для пациентов без факторов риска доза заместительной гормональной терапии составляет 1,6 мкг/кг/сут. Для пожилых пациентов, пациентов с коморбидностями и низкой массой тела титрование дозы гормонов начинают с 25–50 мкг [13]. Токсичность 3–й и 4–й степени следует лечить как степень 2 при отсутствии признаков микседемы (нарушение ментального статуса, гипотония, гипогликемия, брадикардия, гипотермия), при наличии микседемы пациентам следует рекомендовать госпитализацию в эндокринологическое отделение [12].
У пациентов с гипертиреозом при токсичности 2–й степени терапию иИКТ следует прекратить до возвращения показателей функции щитовидной железы к норме. К дополнительной терапии для облегчения симптомов гипертиреоза относят b–блокаторы. Следует отметить, что ГК обычно не требуются для лечения гипертиреоза, ассоциированного с иИКТ. В случае развития персистирующего гипертиреоза (> 6 недель) или клинических подозрений в пользу болезни Грейвса в терапии следует рассмотреть тиреоид–стимулирующий иммуноглобулин или антитела к рецептору ТТГ и тио–амиды (метимазол или пропилтиоурацил) [40]. При развитии токсичности 4–й степени с жизнеугрожающими симптомами или при тиреотоксическом кризе следует госпитализировать пациента и начать терапию преднизолоном 1–2 мг/кг/сут, насыщенным раствором йодида калия или тиоамидами [13]. В целом ТТГ следует контролировать каждые 2–3 недели во время титрования заместительной гормональной терапии до достижения нормального уровня ТТГ с повторным последующим тестированием ежегодно или в соответствии с клиническими показаниями [13].
Поражение надпочечников
При подозрении на первичную надпочечниковую недостаточность рекомендуемое диагностическое обследование включает оценку уровней АКТГ, кортизола, электролитов (Na+, K+) и глюкозы, проведение КТ надпочечников на предмет исключения метастазирования/кровоизлияния [41]. До нормализации уровня гормонов и стабилизации состояния пациентов следует воздержаться от терапии иИКТ при любом уровне токсичности. Для заместительной терапии первичной надпочечниковой недостаточности при 1–й степени токсичности следует рассмотреть преднизолон (5–10 мг/сут) или гидрокортизон (10–20 мг перорально утром, 5–10 мг перорально в полдень) или флудрокортизон (0,1 мг/сут) с титрованием дозы в зависимости от уровня симптомов. При 2–й степени токсичности дозы гормонов возрастают: преднизолон — 20 мг/сут, гидрокортизон — 20–30 мг утром и 10–20 мг днем со снижением до поддерживающих доз в течение 5–10 дней [12]. При 3–4–й степени токсичности пациента необходимо госпитализировать в отделение неотложной помощи с введением ГК внутривенно (пульс–терапия) со снижением до поддерживающих доз в течение 7–14 дней после стабилизации состояния [42].
Поражение поджелудочной железы
Редкие случаи развития сахарного диабета 1–го типа (СД1) на фоне иИКТ представляют собой актуальную проблему для клинициста в необходимости раннего выявления и дифференциации от гораздо более распространенных случаев нарушения гликемического контроля, связанного с метаболическим синдромом и СД2 [36]. Факт развития иИКТ–ассоциированного СД1 требует бдительности со стороны онкологов, несмотря на очень низкую частоту возникновения. Впервые выявленная гипергликемия у пациентов без факторов риска развития СД2 (например, ранее существовавшее заболевание, прием ГК, отсутствие семейного анамнеза, отсутствие ожирения и др.) должна повысить уровень беспокойства лечащего врача по поводу СД1. Острое начало полиурии, полидипсии, снижение массы тела, нарушения сознания являются характерными признаками СД1. Необходимо обследовать уровень кетонов мочи и кислотно–щелочной статус, уровни инсулина и С–пептида для подтверждения диагноза, однако начало терапии не следует откладывать до получения результатов [13]. Консультация эндокринолога необходима при подозрении на СД1 даже без признаков диабетического кетоацидоза для установления диагноза и назначения корректной дозы инсулина.
Ревматологические иммунозависимые побочные эффекты
Данные о частоте ревматических осложнений сильно варьируют. Так, частота артралгии колеблется от 1 до 43 %, а диапазон других проявлений составляет 0,7–5,1 % [43].
Артралгия и артрит
Артралгия и артрит являются наиболее часто встречающимися ревматическими ИЗПЭ, частота которых составляет приблизительно 40 % [44]. ИИКТ–индуцированный артрит может иметь различное время манифестации от момента начала лечения: в среднем симптомы появляются через 5 месяцев (диапазон 1–24 месяца) [45]. Клинические проявления артрита варьируют в клинических наблюдениях в виде олигоартритов, полиартритов и реактивных артритоподобных проявлений [46]. L. Cappelli и соавт. (2016) [46] сообщили, что у пациентов, получавших комбинированную терапию иИКТ, с большей вероятностью вовлекались крупные суставы в сочетании с другими ИЗПЭ, в то время как пациенты, получавшие монотерапию, чаще имели поражение мелких суставов и артрит был единственным ИЗПЭ [46]. Эти пациенты также могут иметь признаки, характерные для реактивного артрита (такие как конъюнктивит или уретрит), и иногда жалуются на боль/скованность в спине или в шее, наводящую на мысль о сакроилеите и спондилите. Также у пациентов можно наблюдать симметричное поражение мелких суставов, напоминающее ревматоидный артрит, кроме того, эти пациенты часто проявляют серопозитивность по ревматоидному фактору и антицитруллинированному циклическому пептиду (анти–ЦЦП) [45]. Без сомнения, такие неожиданные клинические сочетания значительно затрудняют проведение дифференциальной диагностики, они требуют тщательной оценки состояния пациента и клинико–лабораторных находок, клинических размышлений, что возможно только при высокой квалификации врача.
Диагностическая оценка. Пациентам должно быть проведено полное обследование костно–мышечной системы. Лабораторные исследования, включая ОАК, СРБ, ревматоидный фактор анти–ЦЦП, ANA и HLA–B27 (человеческий лейкоцитарный антиген B–27), могут помочь дифференцировать фенотипы артрита с целью индивидуального подхода к лечению. Несмотря на то что большинство пациентов являются серонегативными, есть одно клиническое наблюдение, описывающее серопозитивную подгруппу больных с иИКТ–индуцированным артритом [47]. Также обязательно должна быть проведена визуализация суставов, включая рентгенографию, УЗИ или МРТ суставов, для оценки наличия выпота, синовита и его пролиферации, эрозивных поражений и тендовагинита.
Менеджмент. Раннее распознавание артрита и консультация ревматолога имеют решающее значение для предотвращения эрозивного повреждения суставов и развития функциональной недостаточности. Лечение иИКТ может быть продолжено при токсичности 1–й степени с назначением анальгетических препаратов и нестероидных противовоспалительных препаратов (НПВП). При токсичности 2–й степени от терапии иИКТ следует воздержаться и возобновить ее при контроле симптомов и дозе преднизолона ≤ 10 мг/сут [11]. Для усиления анальгезирующего эффекта следует рассмотреть возможность назначения более высоких доз НПВП, при их неэффективности следует назначать перорально преднизолон 10–20 мг/сут в течение 4–6 недель с последующим постепенным снижением дозы в течение 1 месяца или внутрисуставное введение ГК при моноолигоартрите [11]. При развитии токсичности 3–4–й степени терапию иИКТ следует прекратить, возможно ее возобновление при улучшении состояния пациента. Следует назначать перорально преднизолон 0,5–1 мг/кг/сут, при отсутствии улучшения через 4 недели или усугублении суставного синдрома необходимо добавить болезнь–модифицирующие антиревматические препараты (БМАРП): биологические (антицитокиновая терапия, терапия ингибиторами рецептора ФНО–a, интерлейкина–6 (ИЛ–6)) или синтетические (метотрексат, лефлуномид) [12]. В стероидно–резистентных случаях общепринятый подход заключается в инициации терапии метотрексатом с эскалацией дозы вплоть до назначения биологических агентов при отсутствии адекватного клинического ответа.
Особенностью ревматических ИЗПЭ, в частности иИКТ–индуцированных воспалительных артритов, является их склонность к стойкости и рецидивированию, несмотря на прекращение терапии иИКТ, что может потребовать длительной иммуномодулирующей терапии от нескольких месяцев до нескольких лет после постановки диагноза [48].
Полимиалгия–подобный синдром
Одним из побочных эффектов иммунотерапии рака является тяжелая миалгия в проксимальных отделах мышц верхних и нижних конечностей, с сопутствующей выраженной слабостью, артралгией без признаков синовита, клинически напоминающей ревматическую полимиалгию [49]. Необходимо отметить, что в реальной практике сам диагноз ревматической полимиалгии требует тщательной дифференциации и исключения онкопатологии.
Диагностическая оценка. Следует проводить дифференциальную диагностику с воспалительным миозитом, фибромиалгией, статин–индуцированной мио–патией и другими видами миопатий. Важным шагом является определение миозит–специфических ферментов, прежде всего креатинфосфокиназы (КФК), альдолазы, АсАТ, для дифференциации с миозитом. Визуализация с помощью МРТ и электромиография (ЭМГ) позволяет оценить признаки миопатии или воспаления мышц.
Менеджмент. При токсичности 2–3–й степени от применения иИКТ следует воздержаться, назначить обезболивающие препараты и/или НПВП при необходимости. При 2–й степени токсичности доза преднизолона составляет < 10 мг, при 3–й — 20 мг/сут [12]. При уменьшении степени выраженности симптомов дозу ГК необходимо снижать через 3–4 недели. При отсутствии улучшения и 4–й степени токсичности наряду с приемом преднизолона рекомендуется использовать стероид–сберегающий агент — метотрексат или ингибитор ИЛ–6 тоцилизумаб [12].
Гигантоклеточный артериит
Недавний анализ Всемирной организации здравоохранения VigiBase обнаружил, что у пациентов, получавших иИКТ, соотношение шансов развития гигантоклеточного артериита (ГКА) в 13 раз выше, чем у пациентов, не получавших эту терапию [50]. В этом исследовании также сообщалось, что среднее время начала этого ИЗПЭ от последнего введения иИКТ составило 55 дней (диапазон — 21–98) с большей предрасположенностью у пожилых пациентов, пациентов европеоидной расы и у больных с меланомой [50]. Симптомы ГКА, вызванные иИКТ, аналогичны симптомам традиционной болезни Хортона, включая головную боль в височной области, привычный подвывих нижней челюсти, потерю монокулярного зрения, необъяснимую лихорадку и слабость.
Диагностическая оценка. Ранняя диагностика имеет жизненно важное значение для предотвращения глазных и цереброваскулярных осложнений ГКА. Ухудшение зрения было зарегистрировано у 28 % пациентов с иИКТ–индуцированным ГКА [50]. Необходимо провести полное обследование — ОАК и СРБ. Биопсия височной артерии является золотым стандартом диагностического теста и обеспечивает точный диагноз, но может быть заменена визуализацией височных артерий при УЗИ и контрастной МРТ [12].
Менеджмент. Пациентам с подозрением на иИКТ–индуцированный ГКА следует проводить лечение в соответствии с рекомендациями по лечению ГКА. В случае отсутствия глазных симптомов лечение включает преднизолон 1 мг/кг/сут (максимальная доза — 60 мг/сут) [11]. Пациентам с угрозой или установленной потерей зрения на момент постановки диагноза следует начинать с внутривенного введения пульс–терапии ГК в течение 3 дней с последующей пероральной терапией высокими дозами стероидов [51]. Высокие дозы пероральных ГК следует назначать в течение 2–4 недель, после чего дозу следует снижать на 10 мг каждые 2 недели, пока не будет достигнута доза 40 мг/день; в дальнейшем рекомендуется более медленный темп схождения с дозы — 5 мг каждые 2 недели до 20 мг/сут [13]. В случае рецидивирующих симптомов ГКА, несмотря на высокие дозы ГК, необходимо добавить БМАРП. Абатацепт продемонстрировал высокую эффективность при иИКТ–ассоциированном ГКА, метотрексат и тоцилизумаб являются альтернативным подходом [52]. Возобновление терапии ингибиторами ИКТ может быть рассмотрено по достижении дозы преднизолона < 10 мг/сут при стойком контроле симптомов и после согласования тактики с ревматологом [11].
Миозит
Миозит является редким осложнением терапии иИКТ, однако его течение отличается агрессивностью, резистентностью и ассоциируется с плохим прогнозом. Миозит чаще встречается при приеме ингибиторов PD–1/PD–L1, чем ипилимумаба [53]. Поражение мышц может реактивироваться в результате ранее существовавшего паранеопластического полимиозита/дерматомиозита или быть миозитом de novo. Основным симптомом воспалительного миозита является слабость, преимущественно в проксимальных отделах мышц конечностей, с невозможностью находиться в вертикальном положении, поднимать руки и передвигаться или выраженным ограничением этих функций [53]. У пациентов с миозитом de novo типичная сыпь, характерная для дерматомиозита, не наблюдается. Миозит может иметь молниеносное некротизирующее течение с рабдомиолизом, способен поражать жизненно важные скелетные мышцы, такие как миокард, дыхательные мышцы, и в этом случае требуется неотложное лечение, чтобы избежать фатальных осложнений [54].
Диагностическая оценка. Лабораторные тесты включают исследования уровня мышечных ферментов трансаминаз (АсАТ, АлАТ), лактатдегидрогеназы (ЛДГ), альдолазы; наиболее специфично повышение КФК и воспалительных маркеров [11]. При подозрении на поражение миокарда необходимо исследовать уровень тропонинов, провести эхокардиографию. Нет никаких доказательств диагностического значения присутствия каких–либо конкретных аутоантител при иИКТ–ассоциированном миозите. На ЭМГ можно выявить типичные для миопатии изменения, на МРТ — сигнал повышенной интенсивности и отек пораженных мышц. Для подтверждения диагноза необходимо проведение биопсии мышц. Дифференциальная диагностика включает синдром хронической усталости, ревматическую полимиалгию, фибромиалгию, побочные явления сопутствующей терапии (например, статины, ГК) и мышечную дистрофию, при которых уровень КФК и воспалительных маркеров нормальный [11].
Менеджмент. Пациентов с подозрением на миозит обязательно должен проконсультировать ревматолог. При развитии токсичности 1–й степени терапию иИКТ можно продолжать. При значимом повышении уровня КФК, прогрессирующей мышечной слабости необходимо рассмотреть назначение пероральных ГК и рассматривать такого пациента, как с уровнем токсичности 2–й степени. При 2–й степени токсичности иИКТ следует отменить; возобновить прием возможно при контроле симптомов, нормализации КФК и дозе преднизолона < 10 мг/сут [11]. При сохранении симптомов, устойчивом повышении КФК в 3 и более раза и вовлечении миокарда доза преднизолона составляет 0,5–1 мг/кг/сут. При развитии токсичности 3–4–й степени иИКТ следует сразу же отменить, пациента госпитализировать, проконсультировать у ревматолога и невропатолога, назначить преднизолон в дозе 1–2 мг/кг, особенно при значительном ограничении подвижности, сердечной и дыхательной недостаточности, дисфагии. При отсутствии эффекта в течение 4–6 недель пациенту следует предложить иммуносупрессивную терапию метотрексатом, азатиоприном или микофенолата мофетилом, также рекомендуется применение плазмафереза и внутривенных иммуноглобулинов в высоких дозах (из расчета 1–2 г/кг) [11]. При 4–й степени токсичности рекомендуется пульс–терапия ГК с последующей реоральной терапией в высоких дозах [11]. Ритуксимаб также можно использовать при миозите и 4–й степени токсичности, но рекомендуется соблюдать осторожность, учитывая его длительный период полувыведения [12].
Другие ревматические ИЗПЭ включают васкулит, синдром сухого глаза, волчаноподобные синдромы. Принципы лечения аналогичны тем, о которых сообщалось для других ревматических синдромов, вы–званных иИКТ. Начиная со 2–й степени токсичности рекомендуется отмена препаратов для лечения рака, назначение ГК и в дальнейшем иммуносупрессивной терапии.
Пациенты с ранее существовавшими аутоиммунными ревматическими заболеваниями могут подвергаться как более высокому риску токсичности развития ИЗПЭ, так и обострениям своего ранее существовавшего заболевания [55]. Тем не менее многие из этих пациентов могут продолжать терапию иИКТ, аутоиммунное заболевание в анамнезе не является абсолютным противопоказанием для назначения иммунотерапии рака. Этим пациентам необходим тщательный мониторинг и мультидисциплинарное ведение, так как они нуждаются в сопутствующем лечении своих ранее существовавших аутоиммунных заболеваний, как только у них развивается ИЗПЭ.
Поражение нервной системы
Неврологические токсические явления — достаточно редкие побочные проявления терапии иИКТ, однако они представляют особый интерес из–за их потенциальной клинической тяжести и фатальных последствий. Эти осложнения охватывают нарушения регуляции как центральной, так и периферической нервной системы (ПНС). Вовлечение центральной нервной системы и ПНС включает довольно широкий спектр неврологических расстройств: энцефалит, асептический менингит, синдром задней обратимой энцефалопатии, поперечный миелит, миастению/миастенический синдром, сенсорно–двигательную невропатию или синдром Гийена — Барре (СГБ), болезненную сенсорную и парасимпатическую невропатии [56]. Пациенты могут страдать от головной боли, изменений психического статуса, двигательного или сенсорного дефицита, ненормального поведения, изменения личности, расстройств речи или двигательных нарушений. Метаанализ 9208 пациентов, получавших терапию иИКТ, показал, что частота неврологических нарушений варьировала от 3,8 до 6,1 % при монотерапии анти–CTLA4 и анти–LD–1 соответственно и до 12,0 % при комбинированной терапии [56]. Случаи ИЗПЭ с поражением ЦНС с высокой степенью достоверности регистрировались с частотой < 1 % [56]. Среднее время манифестации энцефалита от момента старта терапии иИКТ составляло 6 недель.
Диагностическая оценка. Первым шагом в менеджменте пациентов является исключение прогрессирования рака с поражением ЦНС, инфекций и метаболических нарушений как причин появившихся неврологических симптомов. Консультация невролога рекомендуется для всех неврологических ИЗПЭ для определения типа и степени неврологических нарушений и тактики дальнейшего ведения пациентов. У пациентов с головной болью, которая изолированно может указывать на асептический менингит, важно оценить наличие нарушения сознания, изменения поведения, афазии, судорожной активности или кратковременной потери памяти, что может указывать на энцефалит [57].
Перечень необходимых исследований включает ОАК, определение функции печени, почек и щитовидной железы, а также оценку состояния гипофиза. При подозрении на васкулит дополнительно следует определять уровни СРБ, ANA и антинейтрофильных цитоплазматических антител. Для исключения анти–NMDA–рецепторного энцефалита рекомендуется определять уровень аутоантител к NR1 и NR2–субъ–единицам глутаматного NMDA–рецептора [58].
МРТ–сканирование головного мозга с контрастированием и электроэнцефалография (ЭЭГ) необходимы для дифференциальной диагностики метастазов в мозг и выявления субклинической судорожной активности. Диагностическое обследование неврологических ИЗПЭ также должно включать МРТ позвоночника и анализ спинномозговой жидкости (СМЖ), проведение цитологического анализа для исключения лептоменингеального метастазирования, инсульта, инфекции. Анализ СМЖ полезен в случаях клинического подозрения на энцефалит, асептический менингит и сенсорно–двигательную невропатию или синдром Гийена — Барре, для которых характерен лимфоцитарный плеоцитоз и повышенный уровень белка. ЭМГ может помочь в диагностике сенсорных симптомов и мышечной слабости [59].
Менеджмент. Быстрая диагностика и оперативный менеджмент ИЗПЭ необходимы для потенциального полного неврологического выздоровления [58]. Первым шагом в лечении является исключение поражения нервной системы вследствие прогрессирования рака, инфекции и метаболических нарушений. При развитии неврологических ИЗПЭ 1–й степени токсичности терапия ИИКТ может быть продолжена под тщательным наблюдением. При неврологических симптомах 2–й степени токсичности и выше от терапии иИКТ следует временно воздержаться до тех пор, пока не будет определена природа ИЗПЭ и отсутствие прогрессирования симптомов. В случае значительной неврологической токсичности 2–й степени или выше следует начинать лечение ГК [11]. При ухудшении состояния для контроля симптомов может потребоваться повышение дозы ГК в дополнение к внутривенному введению иммуноглобулинов или плазмафарезу. Пиридостигмин может быть полезен при миастении в дополнение к терапии ГК [57]. Недавняя серия случаев из 9 пациентов с неврологической токсичностью, вызванной иИКТ, продемонстрировала значительное симптоматическое улучшение у 77,8 % пациентов после прекращения иммунотерапии и лечения ГК в дозе 1–4 мг/кг или пульс–терапии метилпреднизолоном в дозе 1000 мг в течение последовательных 5 дней [58]. В стероид–рефрактерных случаях могут рассматриваться дополнительные терапевтические стратегии: внутривенный иммуноглобулин, ритуксимаб или плазмаферез, поскольку эта токсичность опосредована образованием аутоантител [12].
Миастения гравис (Myasthenia gravis)
По экспертным оценкам, миастения встречается у 0,1–0,2 % пациентов, получающих иммунотерапию [60]. Это клиническое проявление обычно манифестирует в течение 2–3 недель от начала лечения иИКТ [61] с интермиттирующими симптомами моторной слабости и усталости, которые часто ассоциированы с глазной и бульбарной дисфункцией.
Диагностическая оценка. Любые подозрения в пользу миастении требуют быстрой диагностики и лечения, учитывая возможность развития дыхательной недостаточности. Необходимо исследовать уровень специфичных для миастении антител к ацетилхолиновым рецепторам и против поперечнополосатых мышц, уровень мышечно–специфических киназ и липопротеина–4 [12]. Пациентам следует назначать электродиагностические исследования, включая тестирование нервно–мышечной передачи с итерационной стимуляцией нерва (repetitive nerve stimulation, RNS) или дрожания, исследование нервной проводимости для исключения невропатии, игольчатую ЭМГ для диагностики миозита и исследование функции внешнего дыхания [11]. В целях дифференциального диагноза с миозитом необходимо определение миозит–специфических ферментов: креатининкиназы, альдолазы, скорости оседания эритроцитов и СРБ. Для исключения вовлечения ЦНС или сопутствующей патологии следует провести МРТ головного мозга и/или позвоночника в зависимости от преобладающих симптомов [11].
Достаточно часто среди ИЗПЭ у пациентов, получавших лечение иИКТ, наблюдалось сосуществование миастении, миозита и миокардита, что подтверждается в исследовании, где была обнаружена 25% распространенность случаев ниволумаб–ассоциированной миастении [62]. Поэтому назначение кардиологического исследования с проведением ЭКГ и трансторакальной эхокардиографии считается оправданным и целесообразным у всех пациентов с мышечной слабостью и миалгией.
Менеджмент. Миастения, вызванная иИКТ, ассоциирована с более высокой частотой миастенического криза, чем идиопатическая миастения [63]. Таким образом, высокий уровень подозрения и быстрое начало терапии ГК в дозе 1–1,5 мг/кг/сут являются обязательными для предотвращения клинического ухудшения, которое может привести к дыхательной недостаточности и смерти [11]. Пациентов с токсичностью 3–й или 4–й степени следует вести в условиях отделения интенсивной терапии, учитывая риск развития острой дыхательной недостаточности. Терапия высокими дозами ГК рекомендуется внутривенным иммуноглобулином в дозе 2 г/кг в течение 5 дней или плазмаферез в течение 5 дней [11]. Пиридостигмин можно титровать для достижения оптимального облегчения симптомов начиная с 30 мг трижды в день и повышая до максимума — 120 мг [12]. Следует избегать назначения лекарственных препаратов, способных усугубить миастению: b–блокаторы, препараты магния внутривенно, фторхинолоны, аминогликозиды и макролиды.
Периферическая невропатия
Периферическая нейротоксичность иИКТ может проявляться как периферическая невропатия, вегетативная невропатия, синдром Гийена — Барре и некротизирующий миозит.
Синдром Гийена — Барре
При подозрении на синдром Гийена — Барре у пациентов, получающих иИКТ, диагностический подход включает следующие этапы: консультация невропатолога, МРТ позвоночника с контрастом или без него для исключение компрессионного поражения и оценки утолщения нервного корешка, проведение люмбальной пункции с оценкой СМЖ с обязательным выполнением цитологического исследования [12]. Высокую диагностическую ценность при СГБ имеет уровень сывороточных антиганглиозидных антител и их подтипов (например, анти–GQ1b для варианта Миллера — Фишера, связанного с атаксией и офтальмоплегией) [11]. При развитии СГБ все классы токсичности требуют отмены иИКТ и проведения неотложной терапии, учитывая, что прогрессирующий СГБ может привести к острой дыхательной недостаточности. Рекомендуется госпитализация в стационар с возможностью быстрого перехода к мониторингу на уровне отделения интенсивной терапии. Терапию проводят внутривенными иммуноглобулинами в дозе 0,4 г/кг/сут в течение 5 дней с общей дозой 2 г/кг или плазмаферез плюс одновременный прием ГК в средних и высоких дозах [13]. Необходимо также проводить постоянный мониторинг оценки функции внешнего дыхания.
Вегетативная невропатия
При развитии вегетативной невропатии на фоне терапии иИКТ необходимо в первую очередь провести скрининг на другие причины вегетативной дисфункции: сахарный диабет, надпочечниковая недостаточность, ВИЧ, парапротеинемия, амилоидоз, ботулизм, паркинсонизм и другие состояния [11], провести электродиагностические исследования для оценки сопутствующей полинейропатии, рассмотреть целесообразность тестирования на антитела, характерные для паранеопластической аутоиммунной вегетативной дисфункции (например, антиганглионарный рецептор ацетилхолина, антинейрональное ядерное антитело типа 1 и антитела к потенциал–управляемым кальциевым каналам N–типа) [13]. Дифференциальная диагностика в данной ситуации всегда сложная. Пациенты с вегетативной невропатией 1–й степени токсичности могут продолжить прием иИКТ под тщательным наблюдением невролога с регулярным контролем симптомов; при развитии токсичности 2–4–й степени от иИКТ следует воздержаться. В лечебной тактике рекомендуется прием преднизолона в дозе 0,5–1 мг/кг при 2–й степени токсичности и пульс–терапия метилпреднизолоном по 1000 мг/сут трижды при 3–4–й степени токсичности с последующим назначением оральных доз ГК [11].
Асептический менингит
Диагностика асептического менингита на фоне приема иИКТ базируется на проведении МРТ головного мозга, исследовании уровня кортизола и АКТГ для исключения надпочечниковой недостаточности, люмбальной пункции с оценкой цитоза в СМЖ, уровня белка и глюкозы, проведении окрашивания по Граму, культурологического анализа, полимеразной цепной реакции (ПЦР) для исключения вирусных инфекций. Для асептического менингита в СМЖ характерно повышенное количество лейкоцитов с нормальным уровнем глюкозы, нормальная культура и окраска по Граму [11]. При развитии иИКТ–индуцированного асептического менингита терапию иИКТ следует отменить. Должна быть рассмотрена эмпирическая противовирусная (ацикловир в/в) и антибактериальная терапия до получения результатов СМЖ. При отрицательных ПЦР и культурологических исследованиях могут быть назначены ГК преднизолон 0,5–1 мг/кг или в/в метилпреднизолон 1 мг/кг при умеренных/тяжелых формах этого побочного эффекта [13].
Энцефалит
На МРТ головного мозга при иИКТ–индуцированном энцефалите в режиме Т2/FLAIR (инверсия–восстановление с ослаблением сигнала от жидкости) можно выявить типичные изменения, которые наблюдаются при аутоиммунных энцефалопатиях или лимбических энцефалитах [13]. В СМЖ характерно повышенное количество лейкоцитов с преобладанием лимфоцитов и повышенное содержание белка [11]. Для исключения субклинической судорожной активности необходимо провести ЭЭГ, а для оценки возможных причин энцефалита проводят анализ ОАК, СРБ, антинейтрофильных цитоплазматических антител (при подозрении на васкулит, патологии щитовидной железы), включая антитела к тиреопероксидазе и тиреоглобулин.
Менеджмент. При развитии иИКТ–индуцированного энцефалита терапию ИИКТ следует отменить. Как и в случае с асептическим менингитом, рекомендуется вводить ацикловир в/в до получения результатов ПЦР [11]. К лечению добавляют МП 1–2 мг/кг, а при наличии тяжелых или прогрессирующих симптомов или зон олигоклональных иммуноглобулинов на электрофореграмме иммуноглобулинов в СМЖ необходимо провести пульс–терапию метилпреднизолоном 1000 мг в/в ежедневно в течение 3–5 дней плюс в/в иммуноглобулины в дозе 2 г/кг в течение 5 дней [12]. В стероид–резистентных случаях терапией выбора являются ритуксимаб и/или плазмаферез [11].
Поперечный миелит
Предпочтительным методом исследования является МРТ позвоночника (с тонкими осевыми срезами в области предполагаемой аномалии) и МРТ головного мозга с контрастом и без него. Необходимо также проведение исследования СМЖ с оценкой зон олигоклональных иммуноглобулинов и онконевральных антител [11]. В анализах крови следует уделить внимание уровню витамина B12, обследовать пациента на ВИЧ, сифилис, определить АNА, ТТГ, аквапорин–4, иммуноглобулин G [11].
Менеджмент. Такой же, как при энцефалите.
Кардиоваскулярная патология
Миокардит является наиболее часто регистрируемым поражением сердца вследствие ИЗПЭ [64]. Сердечно–сосудистые осложнения в результате терапии иИКТ могут быть потенциально смертельными [50]. D. Wang и соавт. (2018) обнаружили, что именно миокардит имеет самый высокий уровень летальности среди всех описанных ИЗПЭ [6]. Это иммунозависимое осложнение может иметь молниеносное и прогрессирующее течение и быть жизнеугрожающим проявлением [6]. Сообщается, что абсолютная частота кардиоваскулярных ИЗПЭ составляет < 1 %, однако истинная частота, вероятно, выше в связи с недо–оценкой кардио–токсичности этой терапии. Недавно J. Salem и соавт. (2018) сообщили, что вероятность развития миокардита у пациентов, получающих иИКТ, была в 11 раз выше, чем у лиц, не получавших эту терапию [50], со средним временем начала через 30 дней после первоначального воздействия препаратов. Риск может быть увеличен при использовании комбинированной терапии. Так, комбинированная терапия ипилимумабом и ниволумабом имела более высокие показатели сердечно–сосудистых осложнений, чем монотерапия ниволумабом (0,28 против 0,06 %) [54]. Клинический спектр проявлений миокардита может варьировать от слабости, боли в груди, острой сердечной недостаточности до нарушения проводимости, желудочковых аритмий, кардиогенного шока и внезапной смерти [65]. Также могут иметь место острая ишемия миокарда, впервые выявленная систолическая дисфункция, перикардит и синдром Такоцубо [66].
Диагностическая оценка. Миокардит характеризуется повышенными уровнями сердечных ферментов (тропонин, промозговой натрийуретический пептид (про–BNP)) с дисфункцией левого желудочка или без нее и с признаками воспаления миокарда по данным МРТ сердца или позитронно–эмиссионной томографии [11]. В случаях неопределенности в диагностике необходимо проведение эндомиокардиальной био–псии, хотя неинвазивным исследованиям отдается предпочтение [11].
Менеджмент. Терапия кардиальной токсичности требует учета степени развития сердечной недостаточности. Эффективное лечение требует тщательного мониторинга с помощью мультимодального терапевтического плана, состоящего из приостановки терапии иИКТ (временный отказ при 1–3–й степени токсичности и полный отказ — при 4–й), высоких доз ГК (1–2 мг/кг преднизолона в сутки) и ранней консультации кардиолога и ревматолога для предотвращения острых кардиоваскулярных событий [67]. В случае развития стероид–резистентного миокардита необходимо к лечению добавить микофенолата мофетил, инфликсимаб или антитимоцитарный глобулин [68]. Однако конкретных данных, демонстрирующих эффективность этих иммуносупрессивных препаратов, нет. Нарушение проводимости является распространенной и потенциально серьезной причиной внезапной смерти, вызванной иИКТ, даже при отсутствии миокардита [69]. В этом случае необходима консультация электрофизиолога для рассмотрения вопроса об имплантации кардиостимулятора или дефибриллятора; решение о продолжении терапии иИКТ должно приниматься совместно с пациентом, кардиологом и онкологом. Недавно была представлена стратегия, которая предлагает базовую оценку сердечно–сосудистой системы для всех пациентов, получающих иИКТ, включая оценку факторов кардиоваскулярного риска, электрокардио–грамму, сердечный тропонин и про–pBNP в дополнение к неинвазивному протоколу наблюдения для пациентов с сердечной недостаточностью [69]. Учитывая риск смертности от этих осложнений, данный подход может быть целесообразным, однако его еще предстоит оценить в проспективных исследованиях.
Поражение почек
Нефрит является наиболее распространенным клиническим проявлением почечной токсичности у пациентов на фоне терапии анти–PD–1/PD–L1 и чаще встречается у лиц с НМРЛ, получавших комбинацию химиотерапии и иммунотерапии, которая в настоящее время является стандартной терапией первой линии для пациентов с прогрессирующим НМРЛ [70]. Существует значительная гетерогенность во времени возникновения повреждения почек: нефротоксичность на фоне терапии CTLA–4 возникает ранее (диапазон — 2–3 месяца) по сравнению с более поздним началом повреждения почек, ассоциированным с применением PD–1 (диапазон — 3–10 месяцев) [71]. Чаще всего при терапии иИКТ возникает острый интерстициальный нефрит [72]. ИИКТ–опосредованное повреждение почек варьирует от 1 до 2 % в монотерапии и 4,5 % — в комбинированной терапии [73], однако исследование R. Wanchoo и соавт. (2017) представило более высокую заболеваемость — в диапазоне от 9,9 до 29 % [74].
Диагностическая оценка. Почечные ИЗПЭ часто протекают бессимптомно, поэтому для своевременного выявления необходим регулярный мониторинг почечных показателей (сывороточный креатинин, электролиты, общий анализ мочи и УЗИ почек) [11]. При иИКТ–индуцированном нефрите можно выявить пиурию (68 %), гематурию (16 %) и/или протеинурию в анализе мочи и эозинофилию (21 %) в ОАК [75]. У симптомных пациентов могут наблюдаться тошнота, рвота, усталость, изменение психического статуса, уменьшение количества мочи и ее прозрачности, периферические отеки или одышка.
Менеджмент. Терапия иИКТ должна быть временно приостановлена для оценки основной причины поражения почек. Если альтернативной причины не выявлено, предполагается, что у пациентов имеется иммуноопосредованная токсичность, при которой от терапии иИКТ следует воздержаться. При 2–й степени токсичности следует назначать преднизолон в дозе 0,5–1 мг/кг/сут, а при 3–й — более высокие дозы в диапазоне 1–2 мг/кг/сут [11]. Дополнительная иммуносупрессия, включая микофенолата мофетил, может рассматриваться в случаях, устойчивых к ГК, и при отсутствии эффекта от терапии ГК в течение 3–5 дней [11]. Биопсия почки не рекомендуется, пока не предпринята попытка лечения кортикостероидами, которые являются основой лечения.
Терапия иИКТ безопасна у пациентов с исходной почечной недостаточностью неиммунного характера (например, нефрэктомия, поликистоз почек, диабетическая и гипертензивная нефропатия) [76]. Ограниченные данные свидетельствуют о том, что риск отторжения почечного аллотрансплантата на фоне терапии анти–CTLA–4 меньше, чем при лечении анти–PD–1 [76].
Поражение органов зрения
Распространенность офтальмологических ИЗПЭ составляет < 1 % среди пациентов, получавших иИКТ. Офтальмологические ИЗПЭ чаще проявляются как увеит и/или синдром сухого глаза [77, 78]. Глазные ИЗПЭ в среднем отмечаются через 2 месяца от начала терапии и чаще ассоциируются с другими сопутствующими ИЗПЭ, поэтому в данном случае клиническое подозрение на другие побочные эффекты иммунотерапии должно быть высоким [78]. Пациенты жалуются на боль в глазах, усиливающуюся при движении, покраснение склер, нарушение зрения, диплопию и светобоязнь. Менее характерные иИКТ–индуцированные поражения глаз включают воспалительную орбитопатию, кератит, хориоидальную неоваскуляризацию, серозную отслойку сетчатки, ретинопатию, нейроретинит и глазную миастению [77]. Однако такие редкие офтальмологические проявления описаны в литературе в связи с приемом иИКТ.
Диагностическая оценка и менеджмент. Обычно большинство глазных ИЗПЭ не требуют прекращения иИКТ и разрешаются при назначении местного лечения. Все пациенты должны быть проконсультированы офтальмологом для исследования глазного дна с оценкой наличия лейкоцитов в передней камере глаза и степени воспаления. Тем не менее при наличии токсичности 2–3–й степени на фоне лечения иИКТ терапию следует временно прекратить [11]. Искусственные слезы, топические ГК и циклоплегические препараты достаточно эффективны в управлении этой токсичностью, но в редких случаях могут потребоваться системные ГК [11]. Нарушения зрения (< 20/40) являются показанием к неотложной офтальмологической помощи для оценки необходимости специализированных вмешательств и прекращения терапии иИКТ до улучшения состояния [11]. Дополнительные терапевтические стратегии включают системные и местные (введение в стекловидное тело) ГК, применение ингибиторов ФНО. В частности, прием инфликсимаба можно рассматривать в стероид–резистентных случаях [79].
Поражение органов дыхания (пневмонит)
Пневмонит, ассоциированный с иИКТ, определяется как развитие новых инфильтратов в легочной паренхиме при визуализации грудной клетки в сочетании с одышкой или другими респираторными симптомами, но в отсутствие признаков инфекции, сердечной дисфункции или прогрессирования опухоли. Клинические проявления поражения легких при применении иИКТ неоднородны, начиная от бессимптомных рентгенологических изменений, болей в груди, кашля или одышки и заканчивая тяжелой дыхательной недостаточностью [80]. Согласно данным исследования J. Naidoo и соавт. (2017), общая частота пневмонитов на фоне лечения иИКТ колеблется от 0 до 10 %, со средним временем до начала 1–3 месяца [81]. Пациенты, получающие комбинированную терапию иИКТ, подвергаются повышенному риску развития поражения легких (10 против 3 % соответственно; р < 0,001), причем симптомы у этих пациентов проявлялись намного раньше [80]. В исследовании причин смертельных токсических эффектов, связанных с иИКТ, пневмониты, ассоциированные с анти–PD–1/PD–L1, обусловливали 35 % всех смертельных исходов [6].
Новые данные, полученные группой из госпиталя Джона Хопкинса, показали, что гистология опухоли также может быть фактором риска развития пневмонитов у пациентов с НМРЛ [82]. Риск развития иИКТ–ассоциированного пневмонита варьирует в зависимости от типа опухоли. По данным метаанализа М. Nishino и соавт. (2016) [83], вероятность развития пневмонита была выше у пациентов с НМРЛ, чем у лиц с меланомой. Кроме того, в этом исследовании пациенты с почечноклеточным раком также были значительно более склонны к развитию пневмонита всех классов токсичности, чем пациенты с меланомой [83]. В дополнение к типичным признакам пневмонита с CTLA–4– и с PD–1/PD–L1–таргетной терапией были ассоциированы саркоидоподобные гранулематозные реакции, включая субплевральные микронодулярные инфильтраты, внутригрудную лимфаденопатию и плевральный выпот [84]. Клинические проявления пневмонита разнообразны и индивидуальны, могут включать кашель, хрипы, слабость, боль в груди или отсутствие симптомов вообще. При различной клинической картине клиницистам целесообразно осознавать возможность возникновения легочных реакций, связанных с ИЗПЭ, поскольку они могут имитировать прогрессирование заболевания.
Диагностическая оценка. Физикальное обследование при пневмонитах может быть очень малоинформативным, и поэтому врачи должны быть бдительными для раннего выявления этого осложнения на фоне лечения иИКТ. Дифференциальная диагностика должна включать респираторную инфекцию, пневмоцистную пневмонию или аспергиллез (особенно при лечении высокими дозами ГК), прогрессирование опухоли, радиационно–индуцированный пневмонит, иИКТ–индуцированный миокардит и/или сердечную недостаточность [11]. Диагностическая оценка базируется на исключении прежде всего инфекционной этиологии — бактериальный посев мокроты, исследования на предмет вирусной этиологии, бактериальный посев крови, сывороточный галактоманнан, компьютерная томография легких, при необходимости — бронхоскопия с бронхоальвеолярным лаважем и биопсия легкого [11]. Последнее исследование, как правило, не обладает высокой диагностической ценностью, но может быть полезно для исключения инфекции или оценки распространения лимфангитической опухоли. КТ грудной клетки с высоким разрешением является предпочтительным методом визуализации. При помощи этого исследования можно выявить помутнение по типу «матового стекла» или очаговые узловатые инфильтраты, преимущественно в нижних долях, при иИКТ–ассоциированных пневмонитах [85]. Кроме того, пациентам следует проводить оценку функции внешнего дыхания и диффузионной способности легких к монооксиду углерода. КТ и спирометрию следует повторить через 3–4 недели после проведенного лечения для определения ее эффективности. Для исключения легочной тромбоэмболии необходимо провести КТ с внутривенным контрастированием.
Менеджмент. Терапия ГК является основой лечения иИКТ–индуцированных пневмонитов, причем у более чем 80 % пациентов разрешение поражения легких происходит на фоне изолированного лечения преднизолоном [84]. Для лечения пневмонита 1–й или 2–й степени токсичности используют низкие дозы ГК (0,5–1 мг/кг/сут) и тщательное наблюдение [11]. Пневмониты с развитием более высокого уровня токсичности требуют высоких доз ГК, иногда в виде пульс–терапии [12]. Требуются также консультации пульмонолога и инфекциониста для исключения инфекционного процесса. В некоторых случаях может назначаться эмпирическая противомикробная терапия при невозможности полного исключения инфекции. У пациентов без клинического улучшения пневмонита в течение 48–72 ч с момента назначения преднизолона должны быть рассмотрены препараты второй линии, которые включают инфликсимаб 5 мг/кг, микофенолата мофетил в/в 1 г два раза в день, внутривенный иммуноглобулин в течение 5 дней или циклофосфамид [12]. Ретроспективные исследования отмечают, что течение 86 % пневмонитов улучшается на фоне лечения ГК, однако вызывает беспокойство очень слабый ответ на дополнительную иммуносупрессию [86].
Гематологические проявления
Гематологические ИЗПЭ, индуцированные анти–PD–1/PD–L1/CTLA–4, включают аутоиммунную гемолитическую анемию, приобретенную тромботическую тромбоцитопению, гемолитический уремический синдром, иммуноопосредованную тромбоцитопению, лимфопению и приобретенную гемофилию [86].
Апластическая анемия
Апластическая анемия встречается примерно у 11 % пациентов, получающих иИКТ, причем с развитием 3–4–й степени токсичности в среднем у 5,4 % (от 1,1 до 17 %) [87]. Анемия может трансформироваться в панцитопению с развитием аутоиммунной апластической и гемолитической анемии и миелодисплазии [88]. Гематологическая токсичность различных иИКТ одинакова.
Диагностическая оценка. Необходимо исключить сопутствующие возможные причины апластической анемии: прием лекарственных препаратов, воздействие радиации, токсинов, перенесенные недавно вирусные инфекции. Обязательными диагностическими исследованиями при развитии анемии у пациентов, получающих иИКТ, являются: ОАК, подсчет ретикулоцитов, вирусологическое исследование (цитомегаловирусная инфекция (ЦМВ), вирус герпеса человека 6, вирус Эпштейна — Барр, парвовирус), уровень витамина В12, фолатов, железа, меди, церулоплазмина, витамина D, исследование функции печени и почек.
Менеджмент. При развитии такого ИЗПЭ, как апластическая анемия, от терапии иИКТ следует воздержаться; пациентам назначают препараты факторов роста, гемотрансфузию, при необходимости с ежедневной лабораторной оценкой [13]. При развитии токсичности 2–4–й степени к терапии добавляют антитимоцитарный глобулин и гранулоцитарный колониестимулирующий фактор [89]. При отсутствии положительной динамики на фоне проведенной терапии к лечению следует добавить иммуносупрессивную терапию (циклоспорин и алемтузумаб) [13].
Аутоиммунная гемолитическая анемия
Диагностическая оценка. У пациентов с развитием аутоиммунной гемолитической анемии на фоне терапии иИКТ необходимо тщательно собрать анамнез на предмет использования лекарственных препаратов, вызывающих гемолиз (рибавирин, рифампин, дапсон, интерферон, цефалоспорины, пенициллины, НПВП, хинин/хинидин, флударабин, ципрофлоксацин, лоразепам и др.), возможных укусов насекомых, пауков или змей накануне, провести скрининг пароксизмальной ночной гемоглобинурии и метгемоглобинемии. Среди дообследований с особым вниманием следует оценить выраженность анемии, наличие макроцитоза и признаков гемолиза: гаптоглобин, билирубин, количество ретикулоцитов, свободный гемоглобин [13]. Рекомендуется исследование панели внутрисосудистой коагуляции, включая протромбиновое время (ПТ) и международное нормализованное отношение (МНО), проведение электрофореза белков и их фракций и оценку наличия криоглобулинемии [13]. Кроме того, следует обратить внимание на уровни витамина B12, фолатов, железа, глюкозо–6–фосфатдегидрогеназы. При отсутствии очевидной причины гематологического поражения необходимо провести цитогенетический анализ костного мозга.
Менеджмент. При развитии гематологической токсичности 1–й степени прием иИКТ может быть продолжен на фоне тщательного клинического наблюдения и лабораторной оценки. При развитии аутоиммунной гемолитической анемии 2–й степени токсичности и выше настоятельно рекомендуется рассмотреть вопрос о постоянном прекращении применения иИКТ [13]. При появлении токсичности рекомендуется начать пероральный прием преднизолона; при 2–й степени токсичности и выше — перейти на внутривенное введение и пульс–терапию метилпреднизолоном; при 3–4–й степени рекомендуется дополнительный прием фолиевой кислоты 1 мг/сут [13]. Следует оценивать необходимость переливания крови в соответствии с существующими рекомендациями — трансфузия минимального количества эритроцитарных единиц, необходимого для уменьшения симптомов анемии или для возврата пациента к безопасному уровню гемоглобина (70–80 г/л) [13]. При отсутствии улучшения или ухудшении состояния на фоне назначения кортикостероидов или выраженных симптомов следует начать прием других иммунодепрессантов, таких как ритуксимаб, иммуноглобулины, циклоспорин А и микофенолата мофетил [13].
Лимфопения
При развитии лимфопении на фоне терапии иИКТ необходимо уделить особое внимание возможным сопутствующим причинам — терапия препаратами, вызывающими деплецию лимфоцитов: флударабин, антитимоцитарный глобулин, цитотоксическая химиотерапия, облучение, аутоиммунное заболевание в анамнезе. Кроме того, необходимо выполнить рент–генографию органов грудной клетки для исключения тимомы, провести бактериологическое исследование для исключения инфекционной этиологии лимфопении (грибковые, вирусные, бактериальные инфекции, особенно ЦМВ/ВИЧ) [13]. Следует отметить, что лимфопения не является редким побочным проявлением терапии иИКТ, и степень лимфопении следует оценивать по количеству CD4.
Менеджмент. При развитии токсичности 1–3–й степени иИКТ возможно продолжать с еженедельным контролем ОАК. При развитии токсичности 4–й степени необходимо инициировать комплексную профилактику Mycobacterium avium, профилактику пневмонии Pneumocystis jirovecii и ЦМВ [13].
Тромбоцитопения
Тромбоцитопения, индуцированная иИКТ, встречается сравнительно редко, по данным исследований, и составляет в среднем 8 % (от 1 до 28 %) [90].
Диагностическая оценка. При развитии тромбоцитопении следует исключить другие возможные этиологические факторы, включая тромбоцитопеническую микроангиопатию, гемолитико–уремический синдром, синдром диссеминированного внутрисосудистого свертывания, миелодиспластический синдром. Необходимо оценить ОАК, уровень активности ADAMTS13, гаптоглобина, количество ретикулоцитов, билирубина, ПТ, активированного частичного тромбопластинового времени (АЧТВ), фибриногена, провести прямой тест на антиглобулин, серологическое исследование на ЦМВ, ВИЧ, вирус гепатита В и С.
Менеджмент. Первым шагом в менеджменте иИКТ–индуцированной тромбоцитопении является высокий индекс подозрений и своевременное распознавание. У большинства пациентов развитие этого ИЗПЭ любой степени токсичности требует прекращения иммунной терапии, консультации гематолога и назначения пероральных ГК и гемотрансфузии при необходимости [13]. Доза преднизолона при 1–2–й степени токсичности составляет 0,5–1 мг/кг/сут. При развитии токсичности 3–4–й степени следует назначить курсы плазмафереза в соответствии с существующими рекомендациями. Количество процедур зависит от клинического прогресса. После первого плазмафереза назначают пульс–терапию метилпреднизолоном по 1000 мг в/в ежедневно в течение 3 последовательных дней с последующим переходом на пероральный прием преднизолона из расчета 1–2 мг/кг/сут [13]. Возможно применение дополнительных методов лечения, таких как в/в иммуноглобулин, рекомендуется однократное введение в дозе 1 г/кг [91]. При неэффективности терапии ГК и в/в иммуноглобулина последующее лечение может включать спленэктомию, применение ритуксимаба, агонистов рецептора тромбопоэтина или более сильную иммуносупрессию циклоспорином A, микофенолата мофетилом и циклофосфамидом [13].
Гемолитико–уремический синдром
Диагностическая оценка. Развитие гемолитико–уремического синдрома (ГУС) на фоне терапии иИКТ является жизнеугрожающим осложнением с высоким риском фатального исхода. Необходимо тщательно собрать анамнез с особым вниманием к приему препаратов высокого риска, оценить ОАК с определением количества ретикулоцитов и среднего объема эритроцитов, а при наличии макроцитоза исследовать уровень витамина В12 и фолатов, обратить внимание на наличие шистоцитов в мазке крови, что имеет решающее значение для диагностики, исследовать уровни креатинина, определить ADAMTS13 (для исключения тромбоцитопенической пурпуры), активность фракций комплемента — C3, C4, CH50 (антитела, ингибирующие комплемент, при подозрении на врожденные мутации генов) [92]. Учитывая, что в 90 % случаев причиной ГУС является инфекция, вызванная бактериями, продуцирующими веротоксин, — энтерогеморрагическим штаммом Escherichia coli (EHEC, серотип O157:H7 и O104:H4) или Shigella dysenteriae, необходимо провести исследования на эти возбудители. Кроме того, диагностический поиск включает проведение скрининга на вирус Эпштейна — Барр, ЦМВ, вирус герпеса человека 6. Для исключения других причин гемолиза следует провести прямой тест на антитела (тест Кумбса), определение гаптоглобина и ЛДГ [13].
Менеджмент. В случаях развития токсичности –1–2–й степени рекомендуется продолжать лечение иИКТ с тщательным клиническим наблюдением и лабораторной оценкой. При развитии токсичности 3–4–й степени препараты необходимо отменить, инициировать терапию экулизумабом 900 мг в неделю № 4, 1200 мг на 5–й неделе и затем 1200 мг каждые 2 недели [13].
Приобретенная гемофилия
Диагностическая оценка у пациента с подозрением на гемофилию, индуцированную терапией иИКТ, включает ОАК с определением числа тромбоцитов, фибриногена, ПТ, АЧТВ, МНО. При использовании иИКТ были описаны коагулопатии, ассоциированные с дефицитом VIII фактора свертывания [93]. Типичным признаком у пациентов с приобретенной гемофилией А является увеличение длительности АЧТВ с нормальным ПТ. Инструментальные методы показаны для верификации места кровотечения.
Менеджмент. При развитии такого ИЗПЭ, как гемофилия, лечение иИКТ следует немедленно прекратить, проконсультироваться с гематологами. При токсичности 1–й степени преднизолон назначают в дозе 0,5–1 мг/кг/сут, 2–4–й — к преднизолону в дозе 1 мг/кг/сут добавляют ритуксимаб (375 мг/м2 в неделю в течение 4 недель) и/или циклофосфамид (от 1 до 2 мг/кг/сут) [13]. При 4–й степени токсичности могут использоваться факторы свертывания (фактор VII, VIII) [13].
Обсуждение
В настоящее время научных данных об иммунопатогенезе специфических ИЗПЭ мало, однако было предложено несколько механизмов, которые варьируют в зависимости от рассматриваемого побочного эффекта. При аутоиммунном миокардите и аутоиммунной дилатационной кардиомиопатии развитие ИЗПЭ обусловлено повышенной активностью Т–клеток, направленных против перекрестно реагирующих антигенов нормальных и раковых клеток [94]. Кроме того, было обнаружено, что нейтрализация PD–1 пути может способствовать развитию ревматоидного артрита и гигантоклеточного артериита на фоне терапии иИКТ [95]. Витилиго, в свою очередь, являясь наиболее распространенным кожным ИЗПЭ у пациентов с меланомой, вероятно, обусловлено наличием общих антигенов на клетках меланомы и меланоцитах [96]. Также в патофизиологии ИЗПЭ участвуют повышенные уровни воспалительных цитокинов. Обнаружено, что терапия как анти–PD–1, так и анти–CTLA–4 стимулирует Th1– и Th17–опосредованные иммунные ответы, что приводит к повышенным уровням циркулирующего интерлейкина–17 и интерферона–g и способствует развитию ИЗПЭ, в частности энтероколита [97]. Понимание патогенеза ипилимумаб–индуцированного энтероколита обосновывает использование таких стероид–сберегающих агентов, как инфликсимаб и ведолизумаб [98].
Другим потенциальным механизмом развития ИЗПЭ является повышение уровня уже существующих аутоантител с развитием иИКТ–индуцированной миастении, аутоиммунной гемолитической анемии, аутоиммунного тиреоидита и сахарного диабета 1–го типа [99, 100].
Наконец, было обнаружено, что терапия анти–CTLA–4 агентами вызывает гипофизит, вероятно, из–за усиленного воспаления, опосредованного комплементом, и из–за прямого связывания анти–CTLA–4 с CTLA–4, экспрессируемого на нормальной ткани гипофиза [101]. Дальнейшее понимание механизмов возникновения ИЗПЭ позволит разработать персонализированные и целевые методы лечения для пациентов, потенциально снижая воздействие длительной иммуносупрессии, тем самым повышая эффективность и снижая токсичность лечения рака.
Изучается также связь ИЗПЭ с эффективностью терапии иИКТ. Как уже было описано, развитие витилиго имеет значительную связь с благоприятными клиническими исходами [20]. Недавнее многоцентровое ретроспективное исследование пациентов с прогрессирующим НМРЛ, получавших монотерапию ниволумабом, показало, что развитие ИЗПЭ является предиктором лучшей выживаемости [102]. Действительно, пациенты, у которых развивались два или более ИЗПЭ, имели лучшую выживаемость, что предполагает механистическую связь между ИЗПЭ и эффективностью иммунотерапии. Эти результаты не получили универсального подтверждения, но, несомненно, ставят вопрос о том, связана ли степень иммунной активации с лучшим терапевтическим эффектом.
Также оценивались факторы риска развития ИЗПЭ. Клинические проявления, сроки и степень тяжести ИЗПЭ, по–видимому, зависят от режима иммунотерапии, причем более высокая частота и тяжесть были продемонстрированы при комбинированной терапии [7, 36, 103]. Пациенты с предшествующим аутоиммунным заболеванием, по–видимому, подвергаются более высокому риску развития ИЗПЭ. Ряд ретроспективных исследований продемонстрировал, что хотя пациенты с ранее существовавшими аутоиммунными заболеваниями могут подвергаться повышенному риску как обострения их аутоиммунного состояния, так и возникновения аутоиммунного заболевания de novo, однако эти состояния не должны быть противопоказанием для лечения иИКТ [104]. Исследования относительно других факторов риска в отношении развития ИЗПЭ отсутствуют. Вероятно, существует генетическая предрасположенность к развитию ИЗПЭ, учитывая роль генетических факторов в развитии аутоиммунных заболеваний. Действительно, у мышей с дефицитом PD–1 отмечалось развитие различных аутоиммунных состояний с различной степенью выраженности [94].
L. Cappelli и соавт. (2018) провели оценку иммуногенетического статуса у пациентов с иИКТ–индуцированными ИЗПЭ. В ходе исследования было установлено, что пациенты европеоидного происхождения с большей вероятностью были положительными в отношении общих аллелей эпитопа HLA–DRB1 [105]. Кроме того, пациенты с меланомой и генным вариантом CTLA–4 1661A>G больше предрасположены к развитию эндокринных ИЗПЭ [106]. Группа из госпиталя Джона Хопкинса недавно продемонстрировала, что при НМРЛ гистологический тип опухоли может быть фактором риска развития иИКТ–индуцированного пневмонита и что развитие этого ИЗПЭ ухудшает выживаемость у пациентов, получающих иммунотерапию [82].
Новая волна исследований в настоящее время направлена на идентификацию биомаркеров развития ИЗПЭ, которые позволят детерминировать пациентов с более высоким риском развития ИЗПЭ. Было отмечено, что воспалительный фенотип может влиять как на терапевтические реакции иИКТ, так и на их риск токсичности [107]. Также полезно учесть исходные профили воспаления, присутствующие у пациента до приема иИКТ, однако эта область требует дополнительных исследований. Еще одна интересная научная находка при изучении развития ИЗПЭ касается микробиоты кишечника: риск как терапевтических, так и побочных эффектов иИКТ может модулироваться микробиомом [108]. Доклинические исследования показали, что бифидобактерии способствуют естественным противоопухолевым иммунным реакциям, возможно, путем сдвига функционирования иммунной системы в сторону преобладания Th1–ответа [109]. Пациенты, получавшие терапию анти–CTLA–4 с преобладанием Bacteroidetes phylum в микробиоте кишечника, имели более низкую частоту колита, индуцированного ипилимумабом [110]. Эти данные показывают, что изменения в желудочно–кишечной флоре влияют как на иммунитет хозяина, так и на риск развития ИЗПЭ. Действительно, различия в микробиоме кишечника пациентов могут объяснять некоторые неоднородности в результатах эффективности иммунотерапии и развитии токсичности у пациентов, получающих терапию иИКТ. В настоящее время изучается ряд диагностических возможностей. Anderson и Rapport (2018) недавно предложили профиль биомаркеров, включающий IL–17/IFN–g/IL–10/TGF–b1/СРБ, полученных на основании результатов лечения ипилимумаб–ассоциированного колита [111]. Другие потенциальные биомаркеры включают измерение активации циркулирующих нейтрофилов с использованием миелопероксидазы/матриксной металлопротеиназы 9/L–селектина [112].
Выводы
Иммунотерапия рака ассоциирована с уникальным профилем побочных эффектов, приводя к значительной заболеваемости и смертности. Иммуноопосредованная токсичность требует быстрой диагностики и немедленного вмешательства для оптимизации клинических результатов и исходов. Поскольку иммунотерапия в настоящее время все шире используется в онкологической практике, чрезвычайно важным является междисциплинарное сотрудничество онкологов с ревматологами, пульмонологами, эндокринологами, гематологами, нефрологами, окулистами и дерматологами для ранней диагностики и оптимального менеджмента пациентов, получающих лечение иИКТ. Для улучшения понимания иммунопатогенеза ИЗПЭ, разработки стратегий профилактики, раннего выявления и целевой терапии необходимы дальнейшие исследования иИКТ–индуцированных иммунозависимых поражений органов и систем.

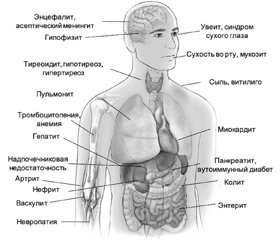
/25-1.jpg)
/26-1.jpg)
/27-1.jpg)
/27-2.jpg)