Международный эндокринологический журнал Том 16, №1, 2020
Вернуться к номеру
Інсулініндукований набряк: рідкісне ускладнення в пацієнта з уперше діагностованим цукровим діабетом 1-го типу (клінічний випадок)
Авторы: H.Y. Kostek(1), N. Köroğlu(2), F.Ö. Çömlek(1), E. Dilek(1), F. Tutunculer(1)
(1) — Department of Pediatrics, Division of Pediatric Endocrinology, Trakya University Faculty of Medicine, Edirne, Turkey
(2) — Department of Pediatrics, Trakya University Faculty of Medicine, Edirne, Turkey
Рубрики: Эндокринология
Разделы: Справочник специалиста
Версия для печати
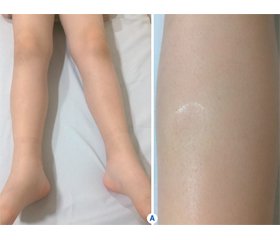
Спричинений введенням інсуліну набряк належить до рідкісних ускладнень інсулінотерапії. Він виникає невдовзі після початку інтенсивної терапії інсуліном у пацієнтів з уперше діагностованим цукровим діабетом (ЦД) 1-го типу або в пацієнтів із погано контрольованим ЦД 2-го типу після початку терапії інсуліном, а також у пацієнтів із низькою масою тіла при введеннi великих доз інсуліну. Процес характеризується розвитком набряку нижніх кінцівок або менш поширеним набряком після введення інсуліну з подальшим спонтанним розсмоктуванням протягом декількох тижнів. Автори повідомляють про випадок розвитку набряку нижніх кінцівок та мошонки в 9-річного хлопчика з уперше діагностованим ЦД 1-го типу через декілька днів після початку лікування інсуліном.
Вызванный введением инсулина отек относится к редким осложнениям инсулинотерапии. Он возникает вскоре после начала интенсивной терапии инсулином у пациентов с впервые диагностированным сахарным диабетом (СД) 1-го типа или у пациентов с плохо контролируемым СД 2-го типа после начала терапии инсулином, а также у пациентов с низкой массой тела при введении больших доз инсулина. Процесс характеризуется развитием отека нижних конечностей или менее распространенным отеком после введения инсулина с последующим самопроизвольным рассасыванием в течение нескольких недель. Авторы сообщают о случае развития отека нижних конечностей и мошонки у 9-летнего мальчика с впервые диагностированным СД 1-го типа через несколько дней после начала лечения инсулином.
Insulin-induced oedema is a rare complication of insulin therapy and occurs shortly after the initiation of intensive insulin therapy in patients with newly diagnosed type 1 diabetes mellitus (DM) or in patients with poorly controlled type 2 DM following the initiation of insulin therapy and also in underweight patients on large doses of insulin. It is characterized by the development of lower extremity oedema or less common generalized oedema after administration of insulin and resolves spontaneously within a few weeks. We report a case of a 9-year-old boy with newly diagnosed type 1 DM, who developed insulin oedema of lower extremities and scrotum within a few days after the initiation of insulin treatment.
набряк; цукровий діабет 1-го типу; інсулін
отек; сахарный диабет 1-го типа; инсулин
oedema; type 1 diabetes mellitus; insulin
Introduction
Case presentation
Discussion
1. Mamoulakis D., Bitsori M., Galanakis E., Raissaki M., Kalmanti M. Insulin induced edema in children and adolescents. J. Paediatr. Child Health. 2006. 42. 655-657.
2. Juliusson P.B., Bjerknes R., Sovik O., Kvistad P.H. Generalized edema following insulin treatment of newly diagnosed diabetes mellitus. Tidsskr. Nor. Laegeforen. 2001. 212. 919-20.
3. Lee P., Kinsella J., Borkman M., Carter J. Bilateral pleural effusions, ascites, and facial and peripheral oedema in a 19-year-old woman 2 weeks following commencement of insulin lispro and detemir — an unusual presentation of insulin oedema. Diabet. Med. 2007. 24. 1282-85.
4. Kalambokis G., Tsatsoulis A., Economou G., Tsianos E.V. A case of insulin edema with inappropriate hyperaldosteronism. J. Endocrinol. Invest. 2004. 27. 957-960.
5. Evans D.J. Pritchard-Jones K., Trotman-Dikenson B. Insulin edema. Postgrad. Med. J. 1986. 62. 665-8.
6. Baş V.N., Cetinkaya S., Agladioglu S.Y., Kendirci H.V.P., Bilgili H., Yıldırım N., Aycan Z. Insulin oedema in newly diagnosed type 1 diabetes mellitus. J. Clin. Res. Pediatr. Endocrinol. 2010. 2. 46-48.
7. Adamo L., Thoelke M. Generalized insulin edema after intensification of treatment with insulin analogues. BMJ Case Report. 2013. doi: 10.1136/bcr-2012-007037.
8. Kalambokis G.N., Tsatsoulis A.A., Tsianos E.V. The edematogenic properties of insulin. Am. J. Kidney Dis. 2004. 44. 579-90.
9. Lawrence J.R., Dunnigan M.G. Diabetic (insulin) oedema. Br. Med. J. 1979. 2. 445.
10. Bulus A.D., Andiran N., Köksal A.O. Insulin edema in type 1 diabetes mellitus: Report of a case and brief review of the literature. Iran J. Pediatr. 2016. 26. e5077.
11. Hopkins D.F., Cotton S.J., Williams G. Effective treatment of insulin-induced edema using ephedrine. Diabetes Care. 1993. 16. 1026-1028.
12. Goturu A., Jain N., Lewis I. Bilateral cataracts and insulin oedema in a child with type 1 diabetes. BMJ Case Reports. 2013. doi: 10.1136/bcr-2012-008235.
13. Aravamudhan A., Gardner C., Smith C., Senniappan S. Insulin oedema in a child with newly diagnosed diabetes mellitus. Eur. J. Pediatr. 2014. 173. 685-687.
14. Zenda T., Murase Y., Yoshida I., Muramoto H., Okada T., Yagi K. Does the use of insulin in a patient with liver dysfunction increase water retention in the body, i.e. cause insulin oedema? Eur. J. Gastroenterol. Hepatol. 2003. 15. 545-549.
15. Spark R.F., Arky R.A., Boulter P.R., Saudek C.D., O’Brian J.T. Renin, aldosterone ad glucagon in the natriuresis of fasting. N. Eng. J. Med. 1975. 292. 1335-1340.
16. Suzuki Y., Kadowaki H., Taniyama M., Kadowaki T., Katagiri H. et al. Insulin edema in diabetes mellitus associated with the 3243 mitochondrial tRNA Leu (UUR) mutation: a case report. Diabetes Res. Clin. Pract. 1995. 29. 137-142.

/104.jpg)