Резюме
Актуальність. Відомо, що дисфункція щитоподібної залози асоційована з підвищеним ризиком розвитку цереброваскулярних захворювань. Однак експериментальні дані останніх років свідчать про те, що гормони щитоподібної залози можуть мати певні нейропротекторні ефекти. Мета дослідження: визначити взаємозв’язок між рівнем вільного трийодтироніну в дебюті гострого ішемічного інсульту та функціональними наслідками інсульту через 6 місяців. Матеріали та методи. У дослідження був включений 121 пацієнт із гострим ішемічним інсультом, що виник вперше. Концентрації вільного трийодтироніну (вT3), вільного тироксину (вT4), тиреотропного гормону та наявність базисних факторів ризику інсульту оцінювалися протягом 24 годин із моменту появи симптомів. Неврологічний дефіцит оцінювався за Скандинавською шкалою інсульту (SSS). Несприятливий функціональний результат визначався як показник mRs ≥ 3 бали через 6 місяців після перенесеного інсульту. Результати. Тест ANOVA показав, що пацієнти з рівнем вT3 в 4-му квартилі (≥ 5,35 пмоль/л) мали значно тяжчий неврологічний дефіцит порівняно з пацієнтами з рівнем вТ3 у 2–3-му квартилях (медіана SSS 48 проти 37 балів, p = 0,0481) і особливо порівняно з пацієнтами з рівнем вТ3 у 1-му квартилі (≤ 3,4050 пмоль/л, медіана SSS 48 проти 30, p = 0,0018). Згідно з тестом Краскала — Уолліса, пацієнти з рівнем вільного Т3 вищим від 75-го процентиля мали більш сприятливий функціональний результат із середнім значенням за модифікованою шкалою Ренкіна 2,5 бала порівняно з пацієнтами з рівнем вT3 в 25–75-му процентилях (із середнім значенням за модифікованою шкалою Ренкіна 4 бали). Результати аналізу пробіт-регресії свідчать про дозозалежний вплив рівня вT3 на ймовірність сприятливих наслідків інсульту. Рівень вільного Т3 6,56 пмоль/л був пов’язаний з 50% ймовірністю сприятливого функціонального результату, а рівень вільного Т3 понад 8,67 пмоль/л був асоційований із 75% імовірністю сприятливого функціонального результату інсульту. Висновки. Дослідження показало, що пацієнти з високим рівнем вільного трийодтироніну при дебюті ішемічного інсульту мають більш сприятливий функціональний результат, навіть порівняно з пацієнтами з нормальним рівнем вільного Т3. Більш високі рівні вільного трийодтироніну також асоціюються з менш тяжким неврологічним дефіцитом у гострій фазі. Виявлено дозозалежний вплив рівня вільного трийодтироніну на ймовірність сприятливого функціонального результату інсульту. Отримані дані свідчать про те, що тиреоїдний профіль є не лише фактором, що впливає на перебіг ішемічного інсульту, але й потенційною мішенню для терапевтичної корекції.
Актуальность. Известно, что дисфункция щитовидной железы ассоциирована с повышенным риском развития цереброваскулярных заболеваний. Однако накопленные в течение последних лет экспериментальные данные свидетельствуют о том, что гормоны щитовидной железы могут иметь определенные нейропротекторные эффекты. Цель исследования: определить взаимосвязь между уровнем свободного трийодтиронина в дебюте острого ишемического инсульта и функциональным исходом инсульта через 6 месяцев. Материалы и методы. В исследование был включен 121 пациент с впервые развившимся острым ишемическим инсультом. Концентрации свободного трийодтиронина (свT3), свободного тироксина (свT4), тиреотропного гормона и наличие базисных факторов риска инсульта оценивались в течение 24 часов с момента появления симптомов. Неврологический дефицит оценивался по Скандинавской шкале инсульта (SSS). Неблагоприятный функциональный исход определялся как показатель mRs ≥ 3 баллов через 6 месяцев после перенесенного инсульта. Результаты. Тест ANOVA показал, что пациенты с уровнем свT3 в 4-м квартиле (≥ 5,35 пмоль/л) имели значительно более тяжелый неврологический дефицит по сравнению с пациентами с уровнем свТ3 во 2–3-м квартилях (медиана SSS 48 баллов против 37 баллов, p = 0,0481) и особенно по сравнению с пациентами с уровнем свТ3 в 1-м квартиле (≤ 3,4050 пмоль/л, медиана SSS 48 против 30, p = 0,0018). Согласно тесту Краскала — Уоллиса, пациенты с уровнем свободного Т3 выше 75-го процентиля имели более благоприятный функциональный исход со средним значением по модифицированной шкале Рэнкина 2,5 балла по сравнению с пациентами с уровнем свT3 в 25–75-м процентилях (со средним значением по модифицированной шкале Рэнкина 4 балла). Результаты анализа пробит-регрессии свидетельствуют о дозозависимом влиянии уровня свT3 на вероятность благоприятного исхода инсульта. Уровень свободного Т3 6,56 пмоль/л был связан с 50% вероятностью благоприятного исхода, а уровень свободного Т3 выше 8,67 пмоль/л был ассоциирован с 75% вероятностью благоприятного исхода инсульта. Выводы. Исследование показало, что пациенты с высоким уровнем свободного трийодтиронина при дебюте ишемического инсульта имеют более благоприятный функциональный исход, даже по сравнению с пациентами с нормальным уровнем свободного Т3. Более высокие уровни свободного трийодтиронина также ассоциируются с менее тяжелым неврологическим дефицитом в острой фазе. Выявлено дозозависимое влияние уровня свободного трийодтиронина на вероятность благоприятного исхода инсульта. Полученные данные свидетельствуют о том, что тиреоидный профиль является не только фактором, влияющим на течение ишемического инсульта, но и потенциальной мишенью для терапевтической коррекции.
Background. Thyroid dysfunction is associated with cerebrovascular diseases. However, there is a growing number of pieces of evidences that thyroid hormones may have certain neuroprotective effects. The purpose of this study was to determine the outcomes of ischemic stroke in patients with various thyroid profiles during stroke onset. Materials and methods. In this study, 121 adult patients with first-ever acute ischemic stroke were enrolled. Concentrations of free T3 (fT3), free T4 (fT4), TSH, and basic stroke risk factors were assessed during 24 h from symptoms onset. The neurologic deficit was assessed by Scandinavian Stroke Scale (SSS). The disabling deficit was defined as mRs score ≥ 3 at 6 months after stroke. Results. ANOVA showed that SSS scores were significantly higher in patients with fT3 level in the 4th quartile (≥ 5.35 pmol/L) compared to the 2nd —3rd quartile (SSS median 48 vs. 37; p = 0.0481) and especially to the 1st quartile (≤ 3.4050 pmol/L, SSS median 48 vs. 30; p = 0.0018). According to the Kruskal-Wallis test, a patient with free T3 level above the 75th percentile has a more favorable outcome with mRs score median of 2.5 in comparison with mRs median of 4 in patients with fT3 level in the 25–75th percentiles. There was also a dose-dependent effect of fT3 level on the probability of favorable stroke outcome. Free T3 level of 6.56 pmol/L was associated with a 50% probability of the favorable outcome, and free T3 level above 8.67 pmol/L was associated with a 75% probability of the favorable stroke outcome. Conclusions. The study showed that patients with high serum free triiodothyronine level during stroke onset experienced more favorable stroke outcome, even in comparison with patients with normal serum free T3 levels. Higher levels of serum free triiodothyronine are also associated with less severe neurologic deficit in the acute phase. There is a dose-dependent effect of free triiodothyronine level on the probability of favorable stroke outcome. These findings suggest that thyroid metabolism is not only a factor impacting the course of ischemic stroke but also a potential target for therapeutic correction.
Abbreviations:
AIS — acute ischemic stroke
ATP — adenosine diphosphate
CRP — C-reactive protein
CI — confidential interval
fT3 — free triiodothyronine
fT4 — free thyroxine
mRs — modified Rankin scale
TSH — thyroid-stimulating hormone
SSS — Scandinavian Stroke Scale
Introduction
Stroke and its consequences is a global medical, social and economic challenge. According to published epidemiological studies, one in four people in the world has or will have a stroke [1]. Indicator of prematurely lost years of a full life (Disability-Adjusted Life Years) according to 2012 data globally amounted to 113 million years [2]. In 32 European countries in 2017 direct economic losses associated with stroke amounted to 60 billion euros [3]. Despite some stabilization of the incidence of stroke in high-income countries, the absolute incidence in the world continues to increase.
The results of several controlled randomized trials of treatment in the hyperacute stroke phase were published in recent years. All studies have confirmed the obvious advantage of an integrated approach in the treatment of AIS caused by thrombotic occlusion of the intracranial arteries of the carotid system. This approach consists in using a combination of systemic thrombolysis and endovascular thrombectomy in the first few hours after the onset of the first symptoms of acute stroke [2, 3]. However, reperfusion therapy is possible only within a relatively narrow therapeutic window [4]. In cases where thrombolysis or thrombectomy is not indicated or there are no opportunities for its implementation, approaches to patient management during the acute period of stroke include both secondary prevention of cerebrovascular disease and an attempt to reduce the severity of neurologic deficit by protecting ischemic (but potentially viable) brain tissue in penumbra zone [5]. Nevertheless, despite the diversity of neuroprotective drugs, different in their mechanism of action and effective in preclinical studies, none of them has enough clinical efficacy. In this regard, the search for new approaches to neuroprotection remains one of the most important tasks of modern neuropharmacology [5].
The survival of the brain tissue under ischemia depends on the intensity of metabolism, oxygen demand, as well as the ability to maintain the redox potential and support the synthesis of high-energy compounds (ATP, etc.). The me-chanism of action of most neuroprotectors is based on the effects on these processes [6, 7].
Over the past decades, special attention had been paid to the neuroprotective properties of endogenous molecules such as VEGF, erythropoietin, brain neurotrophic factor, etc. It is known that triiodothyronine, an active form of the thyroid hormone thyroxin, separates tissue respiration and oxidative phosphorylation. This process leads to disruption of the Krebs cycle, reduced ATP production, hyperthermia, and has a potentially negative effect in acute cerebral ische-mia [8]. On the other hand, triiodothyronine is known to have several neuroprotective effects: it contributes to the uptake of neurotoxic glutamate by astrocytes, stimulation of the Na+/K+ membrane channels in neurons, the restoration of intracellular pH [9]. Thus, selective therapeutic effect on thyroid metabolism (stimulation or inhibition of the function of thyroid hormones) may be a promising potential target for new approaches to the treatment of stroke.
In recent years, more publications appeared in the lite-rature about the possible effect of thyroid hormones on the risk of development, severity, and outcome of acute ischemic stroke. Nevertheless, the results of the published works are rather contradictory [10]. Finally, the nature of the influence of hyper- or hypothyroidism on the course and outcome of a stroke is still unclear.
The purpose of this study was to determine the outcomes of ischemic stroke in patients with various thyroid profiles during stroke onset.
Materials and methods
This study was conducted at the single clinical and research center — V.K. Gusak Institute of Urgent and Reparative Surgery. This study enrolled 121 patients (women — 69, men — 52) aged 42 to 78 years with first-ever acute ischemic heterogeneous stroke. Patients with verified autoimmune thyroiditis or malignancy were excluded from the study. Within 24 hours from stroke onset, basic stroke risk factors were analyzed. Serum free triiodothyronine (fT3), free thyroxine (fT4), and the thyroid-stimulating hormone (TSH) were determined using the ELISA method (ChemWell EIA analyzer with DRG International assay kits). Blood sampling was performed within 24 hours from stroke onset. The neurologic deficit was assessed using Scandinavian Stroke Scale (SSS). Poor stroke outcome was assumed as 3 or more points on the modified Rankin Scale (mRs) after 6 months from stroke onset. Thyroid hormones and TSH levels below the 25th and above the 75th percentiles were assumed as “low” and “high”, respectively.
Statistical analysis was performed using MedCalc v14 software. Continuous data with non-normal distribution is presented as median and 95% CI. For analyzing the variation of neurologic deficit, the ANOVA method was used and patients were divided into subgroups by T3 levels: “hypothyroid” (T3 below the 25th percentile), “euthyroid” (T3 in the 25–75th percentile), “hyperthyroid” (T3 above the 75th percentile). For determining the impact of thyroid hormones on stroke outcome using logistic regression, the patients were dichotomized into subgroups with good outcomes (mRs 0–2) and poor outcomes (mRs 3–6).
Results
Strokes in the carotid territory were the most frequent (71 % of all patients), with the atherothrombotic subtype being the most common (66 %). The leading basic risk factors were arterial hypertension (66 %), coronary heart disease (24 %), atrial fibrillation (29 %).
According to the laboratory reference indicators, 63 patients had thyroid hormone levels in the reference range, 34 patients had laboratory hypothyroidism, and 24 patients had laboratory hyperthyroidism. The distribution of thyroid hormone levels is presented in Table 1.
According to previous clinical studies, including our studies, low serum free triiodothyronine was considered a factor for poor stroke outcome [13, 14]. So, we divi-ded enrolled patients into subgroups according to free T3 levels in stroke onset to determine possible differences in the demographic characteristics in these subgroups. The demographic characteristics of the examined patients, according to free T3 levels at stroke onset, are presented in Table 2.
According to the statistical analysis, male patients with stroke prevailed in the euthyroid and high T3 group, but there were no other statistically significant differences in demographic data.
The correlation analysis using the Spearman rank correlation method revealed a positive statistically significant relationship between the level of free triiodothyronine and the severity of neurologic deficit on the SSS scale. A statistically significant relationship was also found between the level of free thyroxine and the presence of atrial fibrillation. Among other factors, an inverse correlation was observed between the level of C-reactive protein and the severity of neurologic deficit on the SSS scale (R = –0.397, p = 0.0004). No other significant relationships were found between thyroid hormones, the neurologic deficit severity, and the presence of basic risk factors for stroke.
The ANOVA test showed that patients with fT3 level in the IV quartile (≥ 5.35 pmol/L; 95% CI 5.01–5.61) had a less severe stroke (greater SSS scores) compared to patients with fT3 level in II–III quartiles (SSS median is 48 points in Q4 vs 37 points in Q2–3; p = 0.0481), and significantly less severe stroke compared to patients with a T3 level in the first quartile (≤ 3.4050 pmol/L, SSS median 48 points in Q4 vs 30 points in Q1; p = 0.0018). The results of the ANOVA test are presented in Figure 1.
The result of the ANOVA test suggests that low triiodothyronine levels are associated with a more severe neurologic deficit, while high levels may have potential neuroprotective effects. There were no statistically significant variations of the SSS score in subgroups of patients by the fT4 or TSH levels.
To determine other cofactors that may affect stroke severity, multivariate regression was used. After adjustment for basic stroke risk factors (AF, hypertension, diabetes mellitus, age, etc.), free T3 level and C-reactive protein level appeared to be independent factors with p = 0.0001 for CRP and p = 0.0020 for free T3. The coefficient of determination R2 (adjusted) was 0.39, reporting a good model fit.
After 6 months, 85 patients were classified as having a stroke with poor outcome and 36 patients had a favorable outcome. Comparative analysis showed that patients with poor outcomes were older, had significantly lower fT3 level, higher CRP levels, and lower freeT3 to free T4 ratio (Table 3).
To clarify the relationship between the severity of neurologic deficit and the level of free triiodothyronine in serum, a regression analysis was performed. The univariate regression analysis showed that in patients with cardioembolic stroke, fT4 favorably influenced the severity of the stroke on the SSS scale (R2 = 0.75; p = 0.0005), but this association diminished after adjustment for other stroke risk factors (age, hypertension, carotid stenosis, glucose level, C-reactive protein, SSS score).
To confirm the independent effect of the fT3 level on the stroke severity and exclude the influence of co-factors, a multivariate regression analysis was performed. The regression model included basic risk stroke factors (age, arterial hypertension, blood pressure on admission, atrial fibrillation, IHD, diabetes mellitus, smoking), C-reactive protein, free thyroxine, TSH, free T3/free T4 ratio. The variables were introduced in the regression model using the forward method.
To assess the impact of the fT3 level on stroke outcome, the multiple logistic regression method was used. Basic stroke risk factors (age, hypertension, blood pressure at admission, atrial fibrillation, coronary artery dise-ase, diabetes mellitus, smoking) and C-reactive protein, free thyroxine, TSH levels, fT3/fT4 ratio were included in the regression model. Lower fT3 levels were independently associated with poor stroke outcome (odds ratio = 0.3408, 95% CI 0.15–0.77).
To compare stroke outcomes in patients with different thyroid profiles, we used the ANOVA method with the Kruskal-Wallis criterion. The patients were also divided into three groups according to the free T3 level: below the 25th percentile, the 25–75th percentiles, above the 75th percentile. The test reported overall differences in mRs score in groups with T = 7.64; p = 0,0175. The Jonckheere-Terpstra trend test was positive with a p-value = 0.00950. There were statistically significant differences in mRs scores between the high T3 group and euthyroid group and between euthyroid and low T3 groups. The median of mRs score was 4 in low T3 and euthyroid groups and 2.5 in a high T3 group. The graphical results of the Kruskal-Wallis test are presented in Figure 2.
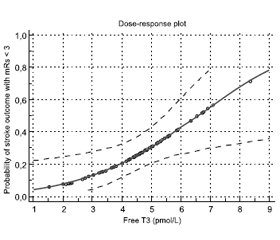
To describe dose-dependent effect of the free T3 level on the probability of a favorable stroke outcome, a probit regression analysis was performed. Dose-dependent effect of the free T3 level on stoke outcome with mRs < 3 score was observed with p = 0.0114. The graphical results of a probit regression analysis are presented in Figure 3.
The findings of a probit regression analysis demonstrated that the free T3 level of 6.56 pmol/L was associated with a 50% probability of a favorable outcome, and the free T3 level above 8.67 pmol/L was associated with a 75% probability of a favorable stroke outcome.
Discussion
The results of this study indicate that low serum free triiodothyronine level during stroke onset is associated with a more severe neurologic deficit in patients with first-ever ischemic stroke and high free triiodothyronine levels are associated with a favorable outcome, even in comparison with euthyroid patients. The difference between this and similar studies is a focus on high free triiodothyronine effects on stroke severity and outcome. There are several limitations in this study. The main disadvantages are lack of long-term follow-up and inability to use the Cox regression method.
Our results correlate with the fundamental researches carried out in recent years. In experimental models, it has been shown that the administration of L-thyroxin after transient cerebral ischemia contributes to an increase in neuron density and stimulation of angiogenesis in the ischemic brain [11]. The results of another in vitro research showed that triiodothyronine can recover intracellular concentration of sodium, calcium ions, and pH [8]. The thyroxine was shown to stimulate the synthesis of other neurotrophic factors, such as fibroblast growth factor [12].
Several clinical studies also confirm the experimental data. L.M. O’Keefe et al. in a study of 868 patients with heterogeneous ischemic stroke found that a low triiodothyronine level was associated with a more functional deficiency 3 months after a stroke and with more often nosocomial mortality [13]. The results of another study of 833 patients with acute ischemic stroke indicate that low levels of total triiodothyronine (even within the reference range) were associated with a poor stroke outcome [14].
In the first hours after disturbance of cerebral blood circulation in neurons in the affected area, ATP pool depletion, inhibition of protein synthesis, and an intracellular pH shift to the acid side are observed. The excessive release of excitatory and inhibitory neurotransmitters, especially glutamate, leads to the development of the excitotoxicity process. The activation of ionotropic and metabotropic glutamate receptors increases the cytosolic level of calcium ions. In turn, an excess of calcium ions disrupts the operation of ion pumps and simultaneously activates many catabolic enzymes, resulting in depolarization and disintegration of the intracellular membranes. The destruction of mitochondrial membranes involves apoptosis due to the release of proapoptotic proteins.
Several experimental studies demonstrated that after the in vivo addition of T3 in astrocyte culture, the number of glutamate transporter proteins GLT-1 and GLAST increased. The activation of glutamate uptake by astrocytes significantly reduced the “gliotoxic” effect of this neurotransmitter on the neurons [23]. Another experiment revealed that triiodothyronine reduced the activity of NMDA receptors in hippocampal neurons, which, according to the authors, prevented glutamate-induced cell death [24].
The key role of iodothyronines in the expression of synthesis and stimulation of ion-exchange pumps has been proved. So, T3 activates the expression of the sodium/hydrogen exchanger gene, which in turn is responsible for removing excess protons and normalizing intracellular pH. Under the action of T3, the number of Na+/K+-ATPases integrated into the membranes increases, the functioning of Ca2+-ATPases increases, which ultimately contributes to the normalization of the ion balance of neurons [25].
Normal functioning of ion pumps is not possible in ATP pool depletion in cells under ischemic conditions. When studying the effect of T3 on astrocyte cultures, an increase in the expression of palmitate beta-oxidation enzymes in mitochondria: beta-hydroxyacyl-CoA dehydrogenase, beta-thiolase, and enoyl-CoA hydratase was recorded [26]. As a result, the amount of ATP increased in astrocytes. Since the protective role of these cells in stroke is considered to be proven, the authors of the study concluded that it was the normalization of energy exchange under the action of triiodothyronine in astrocytes that significantly reduced the lesion area in experimental models of transient cerebral ische-mia and stroke.
A comparison of two experimental models of ischemic stroke — transient and with constant occlusion revealed ano-ther important feature of the neuroprotective effect of T3 — inhibition of protein synthesis of aquaporin-4 with transient occlusion [27]. As a result of the treatment of experimental animals with triiodothyronine preparations, a marked decrease in swelling and the area of brain damage was observed, the probability of a favorable outcome increased but only in the model of transient ischemia.
It is known that iodothyronines regulate metabolic processes by binding not only to nuclear receptors (a classical genomic mechanism) but also to membrane receptors localized on the αVβ3 integrin protein (non-genomic mechanism) [28, 29]. After the hormone binds to a receptor on the integrin surface, the signal is transmitted to an enzyme from the family of mitogen-activated protein kinases — MAP-kinase extracellular signal-regulated kinase 2. Next, some proteins are activated, including the secretion of the basic fibroblast growth factor, bFGF [33]. The study of bFGF function in modeling transient global cerebral ischemia revealed a significant neuroprotective effect [34]. Injections of the basic fibroblast growth factor suppressed the processes of autophagy of neurons and reduced the rate of apoptosis by inhibiting the translocation of the p53 protein into the mitochondria, as a result of which the number of surviving hippocampal neurons increased. Therefore, activation of bFGF secretion by triiodothyronine through the αVβ3 integrin receptors, which are located in the brain and cerebral vascular endothelium, should also have a neuroprotective effect [35].
Even though in recent years, more and more evidence has been obtained of the influence of thyroid hormones on the risk, course, and outcome of cerebrovascular disease, the nature and biological basis of this relationship have not been finally identified. This was the basis to initiate in our Institute a complex clinical and experimental trial devoted to the study of the possibility of using thyroid hormones and their metabolites as neuroprotective therapy in acute ischemic stroke.
Conclusions
The study showed that patients with high serum free triiodothyronine level during stroke onset have a more favorable stroke outcome, even in comparison with patients with normal serum free T3 levels. Higher levels of serum free triiodothyronine are also associated with a less severe neurologic deficit in the acute phase. There is a dose-dependent effect of free triiodothyronine level on the pro-bability of a favorable stroke outcome. These findings suggest that thyroid metabolism is not only a factor impacting the course of ischemic stroke but also a potential target for therapeutic correction. It is reasonable to monitor thyroid hormone levels during a stroke, while the analysis of serum free triiodothyronine can be used to predict a high risk of an unfavorable stroke outcome. There is a need to conduct further clinical trials to determine the safety and efficacy of additional supplements of synthetic triiodothyronine analogs in reducing stroke severity and risk of stroke unfavo-rable outcomes.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Stroke Association. State of the Nation: Stroke Statistics 2015. State of the Nation [Internet]. 2015 (January). 39. Available from: www.stroke.org.uk/sites/default/files/stroke_statistics_2015.pdf.
2. IST-3 collaborative group, Sandercock P., Wardlaw J.M., Lindley R.I., Dennis M., Cohen G. et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet [Internet]. 2012 Jun 23. 379(9834). 2352-63 [cited 2020 Feb 16]. Available from: www.ncbi.nlm.nih.gov/pubmed/22632908.
3. Bhaskar S., Stanwell P., Cordato D., Attia J., Levi C. Reperfusion therapy in acute ischemic stroke: Dawn of a new era? [Internet]. BMC Neurology. 2018. Vol. 18. 8. [cited 2020 Feb 16]. Available from: https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-017-1007-y.
4. Boyle K., Joundi R.A., Aviv R.I. An historical and contemporary review of endovascular therapy for acute ischemic stroke. Neurovascular Imaging [Internet]. 2017 Dec 27. 3(1). 1 [cited 2020 Feb 16]. Available from: http://nvijournal.biomedcentral.com/articles/10.1186/s40809-016-0025-2
5. Liu R., Yuan H., Yuan F., Yang S.H. Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke [Internet]. Neurological Research. Taylor & Francis. 2012. Vol. 34. 331-7 [cited 2020 Feb 16]. Available from: www.tandfonline.com/doi/full/10.1179/1743132812Y.0000000020
6. Ramos-Cabrer P., Campos F., Sobrino T., Castillo J. Targeting the ischemic penumbra. Stroke. 2011. 2442-56.
7. Boltze J., Kleinschnitz C., Reymann K.G., Reiser G., Wagner D.-C., Kranz A. et al. Neurovascular pathophysiology in cerebral ischemia, dementia and the ageing brain — current trends in basic, translational and clinical research. Exp. Transl. Stroke Med. [Internet]. 2012 Jan. 4(1). 14 [cited 2015 Mar 23]. Available from: www.etsmjournal.com/content/4/1/14.
8. Lin H.-Y., Davis F.B., Luidens M.K., Mousa S.A., Cao J.H., Zhou M. et al. Molecular Basis for Certain Neuroprotective Effects of Thyroid Hormone. Front. Mol. Neurosci. [Internet]. 2011. 4. 29 [cited 2017 Mar 19]. Available from: http://journal.frontiersin.org/article/10.3389/fnmol.2011.00029/abstract
9. Bunevicius A., Iervasi G., Bunevicius R. Neuroprotective actions of thyroid hormones and low-T3 syndrome as a biomarker in acute cerebrovascular disorders. Expert. Rev. Neurother. [Internet]. 2015 Mar 4. 15(3). 315-26 [cited 2017 Mar 19]. Available from: www.ncbi.nlm.nih.gov/pubmed/25673072
10. Forti P., Maioli F., Coveri M., Nativio V., Arnone G., Loreti A. et al. Thyroid function tests and early outcomes of acute ischemic stroke in older euthyroid patients. Exp. Gerontol. [Internet]. 2015 Jan. 61. 8-14 [cited 2017 Apr 5]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0531556514003106
11. Schlenker E.H., Schultz H.D. Hypothyroidism stimulates D2 receptor-mediated breathing in response to acute hypoxia and alters D2 receptors levels in carotid bodies and brain. Respir. Physiol. Neurobiol. [Internet]. 2012 Jan 15. 180(1). 69-78 [cited 2017 Sep 15]. Available from: www.ncbi.nlm.nih.gov/pubmed/22051191
12. Sadana P., Coughlin L., Burke J., Woods R., Mdzinarishvili A. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: Possible association with AQP4 modulation. J. Neurol. Sci. 2015. 354(1–2). 37-45.
13. O’Keefe L.M., Conway S.E., Czap A., Malchoff C.D., Benashski S., Fortunato G. et al. Thyroid hormones and functional outcomes after ischemic stroke. Thyroid Res. [Internet]. 2015. 8(1). 9 [cited 2017 Mar 19]. Available from: www.ncbi.nlm.nih.gov/pubmed/26157487.
14. Xu X.Y., Li W.Y., Hu X.Y. Alteration of Thyroid-Related Hormones within Normal Ranges and Early Functional Outcomes in Patients with Acute Ischemic Stroke. Int. J. Endocrinol. [Internet]. 2016. 2016. 1-5 [cited 2017 Sep 15]. Available from: www.ncbi.nlm.nih.gov/pubmed/27375741.
15. Scanlan T.S., Suchland K.L., Hart M.E., Chiellini G., Huang Y., Kruzich P.J. et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. [Internet]. 2004 Jun. 10(6). 638-42 [cited 2017 Sep 15]. Available from: www.ncbi.nlm.nih.gov/pubmed/15146179
16. Venditti P., Napolitano G., Di Stefano L., Chiellini G., Zucchi R., Scanlan T.S. et al. Effects of the thyroid hormone derivatives 3-iodothyronamine and thyronamine on rat liver oxidative capacity. Mol. Cell. Endocrinol. 2011. 341(1–2). 55-62.
17. Piehl S., Hoefig C.S., Scanlan T.S., Köhrle J. Thyro-namines — Past, present, and future. Endocr. Rev. 2011. 32(1). 64-80.
18. Cichero E., Tonelli M. New insights into the structure of the trace amine-associated receptor 2: Homology modelling studies explo-ring the binding mode of 3-iodothyronamine. Chem. Biol. Drug Des. 2017. 89(5). 790-6.
19. Chiellini G., Frascarelli S., Ghelardoni S., Carnicelli V., Tobias S.C., DeBarber A. et al. Cardiac effects of 3-iodothyronamine: A new aminergic system modulating cardiac function. FASEB J. 2007. 21(7). 1597-608.
20. Kinne A., Kleinau G., Hoefig C.S., Grüters A., Köhrle J., Krause G. et al. Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J. Biol. Chem. [Internet]. 2010 Sep 3. 285(36). 28054-63 [cited 2019 Sep 22]. Available from: www.ncbi.nlm.nih.gov/pubmed/20628049.
21. Panas H.N., Lynch L.J., Vallender E.J., Xie Z., Chen G.L., Lynn S.K. et al. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J. Neurosci. Res. 2010. 88(9). 1962-9.
22. Doyle K.P., Suchland K.L., Ciesielski T.M.P., Lessov N.S., Grandy D.K., Scanlan T.S. et al. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke [Internet]. 2007. 38(9). 2569-76 [cited 2017 Sep 15]. Available from: http://stroke.ahajournals.org/content/strokeaha/38/9/2569.full.pdf.
23. Mendes-de-Aguiar C.B., Alchini R., Decker H., Alvarez-Silva M., Tasca C.I., Trentin A.G. Thyroid hormone increases astrocyte glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J. Neurosci. Res. 2008. 86. 3117-3125.
24. Losi G., Garzon G., Puia G. Nongenomic regulation of glutamatergic neurotransmission in hippocampus by thyroid hormones. Neuroscience. 2008. 151. 155-163.
25. Lin H.-Y., Davis F.B., Luidens M.K., Mousa S.A., Cao J.H., Zhou M. et al. Molecular basis for certain neuroprotective effects of thyroid hormone. Frontiers in Molecular Neuroscience. 2011 Oct. Vol. 4. 29.
26. Sayre N.L., Sifuentes M., Holstein D., Cheng S.-Y., Zhu X., Lechleiter J.D. Stimulation of astrocyte fatty acid oxidation by thyroid hormone is protective against ischemic stroke-induced damage. Journal of Cerebral Blood Flow & Metabolism. 2017. Vol. 37(2). 514-52.
27. Sadana P., Coughlin L., Burke J., Woods R., Mdzinarishvili A. Anti-edema action of thyroid hormone in MCAO model of ische-mic brain stroke: possible association with AQP4 modulation. Journal of the Neurological Sciences. 2015. doi: 10.1016/j.jns.2015.04.04.
28. Lin H.-Y. et al. Molecular Basis for Certain Neuroprotective Effects of Thyroid Hormone. Front. Mol. Neurosci. 2011. Vol. 4. 1-6.
29. Sayre N.L. et al. Stimulation of astrocyte fatty acid oxidation by thyroid hormone is protective against ischemic stroke-induced damage. J. Cereb. Blood Flow Metab. 2017. Vol. 37, № 2. 514-527.
30. Sadana P. et al. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: Possible association with AQP4 modulation. J. Neurol. Sci. 2015. Vol. 354, № 1–2. 37-45.
31. Глушаков Р.И., Прошин С.Н., Тапильская Н.И. Роль тиреоидных гормонов в регуляции ангиогенеза, клеточной пролиферации и миграции. Гены & Клетки. 2011. Vol. 6, № 4. 26-33.
32. Lin H.Y. et al. Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discovery Medicine. 2011.
33. Davis P., Davis F., Mousa S. Thyroid Hormone-Induced Angiogenesis. Curr. Cardiol. Rev. 2009. Vol. 5, № 1. 12-16.
34. Sun D. et al. BFGF plays a neuroprotective role by suppressing excessive autophagy and apoptosis after transient global cerebral ischemia in rats. Cell Death Dis. 2018. Vol. 9, № 2.
35. Wu X., Reddy D.S. Integrins as receptor targets for neurological disorders. Pharmacol. Ther. 2012. Vol. 134, № 1. 68-81.
/46.jpg)

/44.jpg)
/45.jpg)
/45_2.jpg)