Международный эндокринологический журнал Том 17, №3, 2021
Вернуться к номеру
Чи належать метаболічний синдром і його компоненти до факторів ризику виникнення поліпів жовчного міхура?
Авторы: Emine Duygu Boz(1), Refik Demirtunç(2), Mehmet Sözen(3)
(1) — Department of İnternal Medicine, Silivri State Hospital, Istanbul, Turkey
(2) — Department of Internal Medicine, Haydarpaşa Numune Training and Research Hospital, Istanbul, Turkey
(3) — Department of Endocrinology and Metabolism, Kocaeli University Faculty of Medicine, Kocaeli, Turkey
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
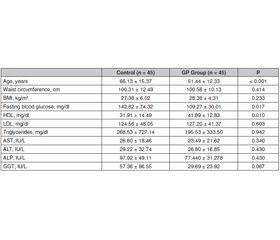
Актуальність. Поліпи жовчного міхура, як правило, є доброякісними утвореннями, що походять із слизової оболонки, і зазвичай виявляються випадково під час рентгенологічних досліджень або після холецистектомії. Поліпи жовчного міхура доволі поширені і можуть мати ризик злоякісного переродження. У цьому дослідженні було встановлено, чи є метаболічний синдром (МС) фактором ризику для виникнення поліпів жовчного міхура. Мета дослідження — визначити поширеність МС та його компонентів у пацієнтів з поліпами жовчного міхура. Матеріали та методи. Проведено ретроспективне перехресне дослідження. Автори дослідили вік, стать та анамнез хвороби у 90 дорослих осіб (45 — з поліпами жовчного міхура, 45 — без поліпів). Ріст та маса тіла, індекс маси тіла, окружність талії та лабораторні дані були отримані з лікарняної системи обробки даних. Для діагностики МС використовували діагностичний критерій МС NCEP-ATP III та Міжнародної діабетичної федерації (IDF). Результати. У дослідженні під спостереженням перебувало 51,1 % (n = 46) жінок і 48,8 % (n = 44) чоловіків. Середній вік становив 58,79 ± 15,70 року. МС виявлено в 56,7 % (n = 51) випадків за критеріями NCEP-ATP III та в 64,4 % (n = 58) випадків за критеріями IDF. У пацієнтів з поліпом жовчного міхура МС виявлено у 55,55 % за критеріями NCEP-ATP III та у 66,66 % за критеріями IDF. Частота МС не була однаковою у групі осіб з поліпами жовчного міхура та контрольній групі (р > 0,01). Встановлено, що абдомінальне ожиріння є фактором ризику розвитку поліпа жовчного міхура (співвідношення шансів 14,23; 95% ДІ: 1,751–15,722; р < 0,01). Хоча показники не досягнули рівня статистичної значущості, низький рівень ліпопротеїнів високої щільності та артеріальна гіпертензія були виявлені приблизно удвічі частіше у пацієнтів з поліпами жовчного міхура, ніж у групі контролю. Висновки. Хоча МС не асоціюється з розвитком поліпа жовчного міхура, ожиріння розглядається як єдиний вірогідний фактор ризику.
Background. Gallbladder polyps are usually benign lesions originating from the mucosa and are usually detected incidentally during radiological examinations or after cholecystectomy. Gallbladder polyps are common and may have malignant risk. In this study, it was investigated whether metabolic syndrome (MS) is a risk factor for gallbladder polyps. This study aimed to determine the prevalence of MS and its components in patients with gallbladder polyps. Materials and methods. We conducted a retrospective, cross-sectional study. We investigated the age, gender and past medical history of 90 adults (45 with polyps, 45 without polyps). Body height and weight, body mass index, waist circumference and laboratory data were obtained from the hospital data processing system. National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) and International Diabetes Foundation (IDF) MS diagnostic criterion were used for the diagnosis of MS. Results. 51.1 % (n = 46) of the subjects participating in the study were female and 48.8 % (n = 44) were male. The mean age was 58.79 ± 15.70 years. MS was found in 56.7 % (n = 51) of the cases according to the criteria of NCEP-ATP III and, in 64.4 % (n = 58) of the cases according to the IDF criteria. In patients with a gallbladder polyp, MS was detected in 55.55 % according to the criteria of NCEP-ATP III and in 66.66 % according to the IDF criteria. The rates of MS were not similar in the gallbladder polyp group and control group (p > 0.01). Abdominal obesity was found to be a risk factor for the development of gallbladder polyp (odds ratio: 14.23, 95% CI: 1.751–15.722; p < 0.01). Although it was not statistically significant, low HDL and hypertension were detected approximately 2 times higher in patients with gallbladder polyps than in the control group. Conclusions. While MS is not associated with the development of gallbladder polyp, obesity is seen as a sole risk factor.
поліп жовчного міхура; метаболічний синдром; абдомінальне ожиріння; фактори ризику
gallbladder polyp; metabolic syndrome; abdominal obesity; risk factors
Introduction
Materials and methods
Results
Discussion
Conclusions
- Bhatt N.R., Gillis A., Smoothey C.O., Awan F.N., Ridgway P.F. Evidence based management of polyps of the gall bladder: a systematic review of the risk factors of malignancy. Surgeon. 2016. 14. 278-286. doi: 10.1016/j.surge.2015.12.001.
- Heitz L., Kratzer W., Gräter T. Gallbladder polyps — a follow-up study after 11 years. BMC Gastroenterol. 2019. 19. 42. https://doi.org/10.1186/s12876-019-0959-3.
- McCain R.S., Diamond A., Jones C., Coleman H.G. Current practices and future prospects for the management of gallbladder polyps: A topical review. World J. Gastroenterol. 2018. 24(26). 2844-2852. doi: 10.3748/wjg.v24.i26.2844.
- Choi Y.S., Do J.H., Seo S.W., Lee S.E., Oh H.C., Min Y.J., Kang H. Prevalence and Risk Factors of Gallbladder Polypoid Lesions in a Healthy Population. Yonsei Med. J. 2016. 57(6). 1370-5. doi: 10.3349/ymj.2016.57.6.1370.
- Lim S.H., Kim D.H., Park M.J., Kim Y.S., Kim C.H., Yim J.Y., Cho K.R., et al. Is Metabolic Syndrome One of the Risk Factors for Gallbladder Polyps Found by Ultrasonography during Health Screening? Gut Liver. 2007. 1(2). 138-44. doi: 10.5009/gnl.2007.1.2.138.
- Huang P.L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2009. 2(5–6). 231-7. doi: 10.1242/dmm.001180.
- Méndez-Sánchez N., Chavez-Tapia N.C., Motola-Kuba D., Sanchez-Lara K., Ponciano-Rodríguez G., Baptista H., Ramos M.H., Uribe M. Metabolic syndrome as a risk factor for gallstone disease. World J. Gastroenterol. 2005. 11(11). 1653-7. doi: 10.3748/wjg.v11.i11.1653.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285(19). 2486-97. doi: 10.1001/jama.285.19.2486.
- Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome — a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006. 23(5). 469-80. doi: 10.1111/j.1464-5491.2006.01858.x.
- Pickering O., Pucher P.H., Toale C., Hand F., Anand E., Cassidy S., McEntee G., Toh S.K.C. Prevalence and Sonographic Detection of Gallbladder Polyps in a Western European Population. J. Surg. Res. 2020. 250. 226-231. doi: 10.1016/j.jss.2020.01.003.
- Babu B.I., Dennison A.R., Garcea G. Management and diagnosis of gallbladder polyps: a systematic review. Langenbecks Arch. Surg. 2015. 400. 455-462. doi: 10.1007/s00423-015-1302-2.
- Gurusamy K.S., Abu-Amara M., Farouk M., Davidson B.R. Cholecystectomy for gallbladder polyp. Cochrane Database Syst. Rev. 2009. 2009(1). CD007052. doi: 10.1002/14651858.CD007052.pub2.
- Wiles R., Thoeni R.F., Barbu S.T., Vashist Y.K., Rafaelsen S.R., Dewhurst C., Arvanitakis M. et al. Management and follow-up of gallbladder polyps : Joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery - European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur. Radiol. 2017. 27(9). 3856-3866. doi: 10.1007/s00330-017-4742-y.
- Park J.K., Yoon Y.B., Kim Y.T., Ryu J.K., Yoon W.J., Lee S.H., Yu S.J. et al. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008. 2(2). 88-94. doi: 10.5009/gnl.2008.2.2.88.
- Ahn D.W., Jeong J.B., Kang J., Kim S.H., Kim J.W., Kim B.G., Lee K.L., et al. Fatty liver is an independent risk factor for gallbladder polyps. World J. Gastroenterol. 2020. 26(44). 6979-6992. doi: 10.3748/wjg.v26.i44.6979.
- Dairi S., Demeusy A., Sill A.M., Patel S.T., Kowdley G.C., Cunningham S.C. Implications of gallbladder cholesterolosis and cholesterol polyps? J. Surg. Res. 2016. 200(2). 467-72. doi: 10.1016/j.jss.2015.08.037.
- Lee Y.J., Park K.S., Cho K.B., Kim E.S., Jang B.K., Chung W.J., Hwang J.S. Shifting prevalence of gallbladder polyps in Korea. J. Korean Med. Sci. 2014. 29(9). 1247-52. doi: 10.3346/jkms.2014.29.9.1247.
- Oh J.S., Lee H.M., Lin T.C., Seo H.N., Kong H.R., Cho S.A., Choi B.G. Relationship between Gallbladder Polyps and Metabolic Syndrome Components in Korean Male Adults. Korean J. Fam. Pract. 2018. 8(1). 21-24. https://doi.org/10.21215/kjfp.2018.8.1.21.
- Lee J.K., Hahn S.J., Kang H.W., Jung J.G., Choi H.S., Lee J.H., Han I.W. et al. Visceral Obesity Is Associated with Gallbladder Polyps. Gut Liver. 2016. 10(1). 133-9. doi: 10.5009/gnl14506.
- Yamin Z., Xuesong B., Guibin Y., Liwei L., Fei L. Risk factors of gallbladder polyps formation in East Asian population: A meta-analysis and systematic review. Asian Journal of Surgery. 2020. 43(1). 52-59. https://doi.org/10.1016/j.asjsur.2019.03.015.
- Li Y., Tejirian T., Collins J.C. Gallbladder Polyps: Real or Imagined? Am. Surg. 2018. 84(10). 1670-1674. PMID: 30747692.
- Lim S.H., Kim D., Kang J.H., Song J.H., Yang S.Y., Yim J.Y., Chung S.J. et al. Hepatic fat, not visceral fat, is associated with gallbladder polyps: a study of 2643 healthy subjects. J. Gastroenterol. Hepatol. 2015. 30(4). 767-74. doi: 10.1111/jgh.12841.
- Stinton L.M., Shaffer E.A. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012. 6(2). 172-87. doi: 10.5009/gnl.2012.6.2.172.
- Shabanzadeh D.M. Incidence of gallstone disease and complications. Curr. Opin. Gastroenterol. 2018. 34(2). 81-89. doi: 10.1097/MOG.0000000000000418.

/31.jpg)
/32.jpg)