Журнал «Медицина неотложных состояний» Том 19, №7, 2023
Вернуться к номеру
Роль нейтрофільних позаклітинних пасток при тромбозі
Авторы: Чуклін С.М., Чуклін С.С.
Медичний центр Святої Параскеви, м. Львів, Україна
Рубрики: Медицина неотложных состояний
Разделы: Справочник специалиста
Версия для печати
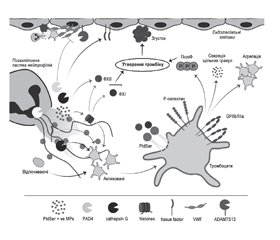
Відповідно до клітинної моделі гемостазу процес згортання крові поданий у вигляді трьох фаз: ініціації, посилення і поширення, кожна з яких включає декілька послідовних стадій. Водночас утворення тромбу часто пояснюють тріадою Virchow: застій кровотоку, пошкодження стінок судин і гіперкоагуляція. За класичними уявленнями, один із трьох згаданих чинників може призвести до тромбоутворення. За останнє десятиліття розширились наші знання про перехресний зв’язок між коагуляцією, запаленням і вродженою імунною активацією і участь нейтрофільних позаклітинних пасток у цих процесах. У даному короткому огляді показана їх роль при тромбозі через механізми активування тромбоцитів, комплементу, взаємодію з факторами згортання крові й пошкодження ендотелію судин. Пошук літератури здійснювався в базі даних MEDLINE на платформі PubMed.
According to the cellular model of hemostasis, the process of blood coagulation is presented in the form of three phases: initiation, amplification and propagation, each of them includes several consecutive stages. At the same time, thrombus formation is often explained by Virchow’s triad: blood stasis, damage to the blood vessel walls, and hypercoagulation. Classically, the appearance of one of the three mentioned parameters can lead to thrombus formation. Over the past decade, our knowledge of the cross-talk between coagulation, inflammation, and innate immune activation and the involvement of neutrophil extracellular traps in these processes has expanded. This brief review shows their role in thrombosis through the mechanisms of activation of platelets, complement, interaction with blood coagulation factors and damage to the vascular endothelium. We searched the literature in the MEDLINE database on the PubMed platform.
тромбоз; нейтрофільні позаклітинні пастки; тромбоцити; комлемент; ендотеліальні клітини; огляд
thrombosis; neutrophil extracellular traps; platelets; complement; endothelial cells; review
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Lozano R., Naghavi M., Foreman K. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012. 380(9859). 2095-2128. doi: 10.1016/S0140-6736(12)61728-0.
- Furie B., Furie B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008. 359(9). 938-949. doi: 10.1056/NEJMra0801082.
- Wienkamp A.K., Erpenbeck L., Rossaint J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022. 13. 953129. doi: 10.3389/fimmu.2022.953129.
- Gross P.L., Furie B.C., Merrill-Skoloff G., Chou J., Furie B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J. Leukoc. Biol. 2005. 78(6). 1318-1326. doi: 10.1189/jlb.0405193.
- Laridan E., Martinod K., De Meyer S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019. 45(1). 86-93. doi: 10.1055/s-0038-1677040.
- de Boer O.J., Li X., Teeling P. et al. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb. Haemost. 2013. 109(2). 290-297. doi: 10.1160/TH12-06-0425.
- Abrams S.T., Zhang N., Manson J. et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013. 187(2). 160-169. 10.1164/rccm.201206-1037OC.
- Fuchs T.A., Kremer Hovinga J.A., Schatzberg D., Wagner D.D., Lämmle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012. 120(6). 1157-1164. doi: 10.1182/blood-2012-02-412197.
- Noubouossie D.F., Reeves B.N., Strahl B.D., Key N.S. Neutrophils: back in the thrombosis spotlight. Blood. 2019. 133(20). 2186-2197. doi: 10.1182/blood-2018-10-862243.
- Brinkmann V., Reichard U., Goosmann C. et al. Neutrophil extracellular traps kill bacteria. Science. 2004. 303(5663). 1532-1535. doi: 10.1126/science.1092385.
- Brinkmann V., Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell. Biol. 2012. 198(5). 773-783. doi: 10.1083/jcb.201203170.
- Pilsczek F.H., Salina D., Poon K.K. et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010. 185(12). 7413-7425. doi: 10.4049/jimmunol.1000675.
- Bergmann S., Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb. Haemost. 2007. 98(3). 512-520. PMID: 17849039.
- Tan C., Aziz M., Wang P. The vitals of NETs. J. Leukoc. Biol. 2021. 110(4). 797-808. doi: 10.1002/JLB.3RU0620-375R.
- Urban C.F., Ermert D., Schmid M. et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009. 5(10). e1000639. doi: 10.1371/journal.ppat.1000639.
- Fuchs T.A., Abed U., Goosmann C. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell. Biol. 2007. 176(2). 231-241. doi: 10.1083/jcb.200606027.
- Yang H., Biermann M.H., Brauner J.M., Liu Y., Zhao Y., Herrmann M. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front. Immunol. 2016. 7. 302. doi: 10.3389/fimmu.2016.00302.
- Carestia A., Kaufman T., Schattner M. Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front. Immunol. 2016. 7. 271. doi: 10.3389/fimmu.2016.00271.
- Fuchs T.A., Brill A., Duerschmied D. et al. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci U S A. 2010. 107(36). 15880-15885. doi: 10.1073/pnas.1005743107.
- Jiménez-Alcázar M., Rangaswamy C., Panda R. et al. Host DNases Prevent Vascular Occlusion by Neutrophil Extracellular Traps. Science. 2017. 358(6367). 1202-1206. doi: 10.1126/science.aam8897.
- Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014. 123(18). 2768-2776. doi: 10.1182/blood-2013-10-463646.
- Oklu R., Albadawi H., Watkins M.T., Monestier M., Sillesen M., Wicky S. Detection of Extracellular Detection of extracellular genomic DNA scaffold in human thrombus: implications for the use of deoxyribonuclease enzymes in thrombolysis. J. Vasc. Interv. Radiol. 2012. 23(5). 712-718. doi: 10.1016/j.jvir.2012.01.072.
- Wang Y., Luo L., Braun O.Ö. et al. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci. Rep. 2018. 8(1). 4020. doi: 10.1038/s41598-018-22156-5.
- Ducroux C., Di Meglio L., Loyau S. et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke. 2018. 49(3). 754-757. 10.1161/STROKEAHA.117.019896.
- Morrissey J.H., Choi S.H., Smith S.A. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012. 119(25). 5972-5979. doi: 10.1182/blood-2012-03-306605.
- Chrysanthopoulou A., Kambas K., Stakos D. et al. Interferon lambda1/IL-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J. Pathol. 2017. 243(1). 111-122. doi: 10.1002/path.4935.
- Elaskalani O., Abdol Razak N.B., Metharom P. Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. Cell. Commun. Signal. 2018. 16(1). 24. doi: 10.1186/s12964-018-0235-0.
- Noubouossie D.F., Whelihan M.F., Yu Y.B. et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017. 129(8). 1021-1029. doi: 10.1182/blood-2016-06-722298.
- Letsiou E., Teixeira Alves L.G., Felten M. et al. Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure. Cells. 2021. 10(12). 3581. doi: 10.3390/cells10123581.
- Xu J., Zhang X., Monestier M., Esmon N.L., Esmon C.T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011. 187(5). 2626-2631. doi: 10.4049/jimmunol.1003930.
- Campos J., Ponomaryov T., De Prendergast A. et al. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 2021. 5(9). 2319-2324. doi: 10.1182/bloodadvances.2020003377.
- Zwaal R.F., Comfurius P., van Deenen L.L. Membrane asymmetry and blood coagulation. Nature. 1977. 268(5618). 358-360. doi: 10.1038/268358a0.
- Fuchs T.A., Bhandari A.A., Wagner D.D. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011. 118(13). 3708-3714. doi: 10.1182/blood-2011-01-332676.
- Maugeri N., Campana L., Gavina M. et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014. 12(12). 2074-2088. doi: 10.1111/jth.12710.
- Stakos D.A., Kambas K., Konstantinidis T. et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015. 36(22). 1405-1414. doi: 10.1093/eurheartj/ehv007.
- Zhang H., Zhou Y., Qu M. et al. Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury. Front. Cell. Infect. Microbiol. 2021. 11. 677902. doi: 10.3389/fcimb.2021.677902.
- von Brühl M.L., Stark K., Steinhart A. et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012. 209(4). 819-835. doi: 10.1084/jem.20112322.
- Stavrou E.X., Fang C., Bane K.L. et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Invest. 2018. 128(3). 944-959. doi: 10.1172/JCI92880.
- Iba T., Miki T., Hashiguchi N., Tabe Y., Nagaoka I. Is the neutrophil a ‘‘prima donna’’ in the procoagulant process during sepsis? Crit. Care. 2014. 18(4). 230. doi: 10.1186/cc13983.
- Reyes-García A.M.L., Aroca A., Arroyo A.B. et al. Neutrophil extracellular trap components increase the expression of coagulation factors. Biomed. Rep. 2019. 10(3). 195-201. doi: 10.3892/br.2019.1187.
- Yang X., Li L., Liu J., Lv B., Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1. Thromb. Res. 2016. 137. 211-218. doi: 10.1016/j.thromres.2015.10.012.
- Gould T.J., Lysov Z., Swystun L.L. et al. Extracellular Histones Increase Tissue Factor Activity and Enhance Thrombin Generation by Human Blood Monocytes. Shock. 2016. 46(6). 655-662. doi: 10.1097/SHK.0000000000000680.
- Semeraro F., Ammollo C.T., Morrissey J.H. et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011. 118(7). 1952-1961. doi: 10.1182/blood-2011-03-343061.
- Massberg S., Grahl L., von Bruehl M.L. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010. 16(8). 887-896. doi: 10.1038/nm.2184.
- Renné T., Stavrou E.X. Roles of Factor XII in Innate Immunity. Front. Immunol. 2019. 10. 2011. doi: 10.3389/fimmu.2019.02011.
- Folco E.J., Mawson T.L., Vromman A. et al. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1a and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018. 38(8). 1901-1912. doi: 10.1161/ATVBAHA.118.311150.
- Komissarov A.A., Florova G., Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J. Biol. Chem. 2011. 286(49). 41949-41962. doi: 10.1074/jbc.M111.301218.
- Gould T.J., Vu T.T., Swystun L.L. et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014. 34(9). 1977-1984. doi: 10.1161/ATVBAHA.114.304114.
- Mai S.H., Khan M., Dwivedi D.J. et al. Delayed but not Early Treatment with DNase Reduces Organ Damage and Improves Outcome in a Murine Model of Sepsis. Shock. 2015. 44(2). 166-172. doi: 10.1097/SHK.0000000000000396.
- Vu T.T., Leslie B.A., Stafford A.R., Zhou J., Fredenburgh J.C., Weitz J.I. Histidine-rich glycoprotein binds DNA and RNA and attenuates their capacity to activate the intrinsic coagulation pathway. Thromb. Haemost. 2016. 115(1). 89-98. doi: 10.1160/TH15-04-0336.
- Gould T.J., Vu T.T., Stafford A.R., Dwivedi D.J. et al. Cell-Free DNA Modulates Clot Structure and Impairs Fibrinolysis in Sepsis. Arterioscler. Thromb. Vasc. Biol. 2015. 35(12). 2544-2553. doi: 10.1161/ATVBAHA.115.306035.
- Longstaff C., Varju I., Sotonyi P. et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 2013. 288(10). 6946-6956. doi: 10.1074/jbc.M112.404301.
- Ammollo C.T., Semeraro F., Xu J., Esmon N.L., Esmon C.T. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011. 9(9). 1795-1803. doi: 10.1111/j.1538-7836.2011.04422.x.
- Grässle S., Huck V., Pappelbaum K.I. et al. Von willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler. Thromb. Vasc. Biol. 2014. 34(7). 1382-1389. doi: 10.1161/ATVBAHA.113.303016.
- Kolaczkowska E., Jenne C.N., Surewaard B.G. et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015. 6. 6673. doi: 10.1038/ncomms7673.
- Brill A., Fuchs T.A., Savchenko A.S. et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 2012. 10(1). 136-144. doi: 10.1111/j.1538-7836.2011.04544.x.
- Staessens S., Denorme F., Francois O. et al. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica. 2020. 105(2). 498-507. doi: 10.3324/haematol.2019.219881.
- Lam F.W., Cruz M.A., Parikh K., Rumbaut R.E. Histones stimulate von Willebrand factor release in vitro and in vivo. Haematologica. 2016. 101(7). e277-279. doi: 10.3324/haematol.2015.140632.
- Saffarzadeh M., Juenemann C., Queisser M.A. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012. 7(2). e32366. doi: 10.1371/journal.pone.0032366.
- Dhananjayan R., Koundinya K.S., Malati T., Kutala V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J. Clin. Biochem. 2016. 31(4). 372-379. doi: 10.1007/s12291-015-0516-y.
- Baselet B., Sonveaux P., Baatout S., Aerts A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019. 76(4). 699-728. doi: 10.1007/s00018-018-2956-z.
- Carmona-Rivera C., Zhao W., Yalavarthi S., Kaplan M.J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 2015. 74(7). 1417-1424. doi: 10.1136/annrheumdis-2013-204837.
- Rabinovitch M. NETs Activate Pulmonary Arterial Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2016. 36(10). 2035-2037. doi: 10.1161/ATVBAHA.116.308206.
- Aldabbous L., Abdul-Salam V., McKinnon T. et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016. 36(10). 2078-2087. doi: 10.1161/ATVBAHA.116.307634.
- Ma Y., Yang X., Chatterjee V., Meegan J.E., Beard R.S. Jr, Yuan S.Y. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front. Immunol. 2019. 10. 1037. doi: 10.3389/fimmu.2019.01037.
- Joffre J., Hellman J., Ince C., Ait-Oufella H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020. 202(3). 361-370. doi: 10.1164/rccm.201910-1911TR.
- Kim J.E., Yoo H.J., Gu J.Y., Kim H.K. Histones Induce the Procoagulant Phenotype of Endothelial Cells through Tissue Factor Up-Regulation and Thrombomodulin Down-Regulation. PLOS One. 2016. 11(6). e0156763. doi: 10.1371/journal.pone.0156763.
- Sugiyama S., Kugiyama K., Aikawa M., Nakamura S., Ogawa H., Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 2004. 24(7). 1309-1314. doi: 10.1161/01.ATV.0000131784.50633.4f.
- Yu S., Liu J., Yan N. Endothelial Dysfunction Induced by Extracellular Neutrophil Traps Plays Important Role in the Occurrence and Treatment of Extracellular Neutrophil Traps-Related Disease. Int. J. Mol. Sci. 2022. 23(10). 5626. doi: 10.3390/ijms23105626.
- Szturmowicz M., Demkow U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021. 22. 8854. doi: 10.3390/ijms22168854.
- Maiocchi S.L., Ku J., Thai T., Chan E., Rees M.D., Tho-mas S.R. Myeloperoxidase: A versatile mediator of endothelial dysfunction and therapeutic target during cardiovascular disease. Pharmacol. Ther. 2021. 221. 107711. doi: 10.1016/j.pharmthera.2020.107711.
- Sorvillo N., Mizurini D.M., Coxon C. et al. Plasma Peptidylarginine Deiminase IV Promotes VWF-Platelet String Formation and Accelerates Thrombosis After Vessel Injury. Circ. Res. 2019. 125(5). 507-519. doi: 10.1161/CIRCRESAHA.118.314571.
- Dong J.F., Moake J.L., Nolasco L. et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002. 100(12). 4033-4039. doi: 10.1182/blood-2002-05-1401.
- Yang J., Wu Z., Long Q. et al. Insights Into Immunothrombosis: The Interplay Among Neutrophil Extracellular Trap, von Willebrand Factor, and ADAMTS13. Front. Immunol. 2020. 11. 610696. doi: 10.3389/fimmu.2020.610696.
- Bonnefoy A., Hantgan R., Legrand C., Frojmovic M.M. A model of platelet aggregation involving multiple interactions of thrombospondin-1, fibrinogen, and GPIIbIIIa receptor. J. Biol. Chem. 2001. 276(8). 5605-5612. doi: 10.1074/jbc.M010091200.
- Bonnefoy A., Daenens K., Feys H.B. et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006. 107(3). 955-964. doi: 10.1182/blood-2004-12-4856.
- Stark K., Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021. 18(9). 666-682. doi: 10.1038/s41569-021-00552-1.
- Van Avondt K., Maegdefessel L., Soehnlein O. Therapeutic Targeting of Neutrophil Extracellular Traps in Atherogenic Inflammation. Thromb. Haemost. 2019. 119(4). 542-552. doi: 10.1055/s-0039-1678664.
- Magro C., Mulvey J.J., Berlin D. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020. 220. 1-13. doi: 10.1016/j.trsl.2020.04.007.
- Wang H., Wang C., Zhao M.H., Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015. 181(3). 518-527. doi: 10.1111/cei.12654.
- de Bont C.M., Boelens W.C., Pruijn G.J.M. NETosis, complement, and coagulation: a triangular relationship. Cell. Mol. Immunol. 2019 Jan. 16(1). 19-27. doi: 10.1038/s41423-018-0024-0.
- Skendros P., Mitsios A., Chrysanthopoulou A. et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 2020. 130(11). 6151-6157. doi: 10.1172/JCI141374.
- Kapoor S., Opneja A., Nayak L. The role of neutrophils in thrombosis. Thromb. Res. 2018. 170. 87-96. doi: 10.1016/j.thromres.2018.08.005.