Журнал «Здоровье ребенка» 1 (69) 2016
Вернуться к номеру
Клиническое значение избыточного содержания лактозы в диете (часть 1)
Авторы: Абатуров А.Е., Никулина А.А. - ГУ «Днепропетровская медицинская академия Министерства здравоохранения Украины»; Демиденко Ю.В. - КУ «Павлоградская городская больница № 4» ДОС
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
В статье на основании литературных источников представлены статистические данные среднегодовых уровней потребления лактозы в странах мирового сообщества, рассмотрено клиническое значение избыточного содержания лактозы в диете в зависимости от полиморфизма гена лактазы. Лактоза — основной источник энергии для детей первых месяцев жизни, который обеспечивает около 40–45 % суточной энергетической потребности организма ребенка. Нарушение всасывания лактозы (мальабсорбция), при дефиците фермента лактазы, сопровождается симптомами лактозной интолерантности. Интерес к изучению иммуномодулирующего влияния β-галактозида лактозы связан с подавлением ею галектина 9 (Gal-9), регулирующего Т-клеточные иммунные реакции с участием Т-хелперных клеток 1-го и 17-го типов (Тh1, Тh17) и Т-регуляторных лимфоцитов (Treg), которые вовлечены во многие иммуноопосредованные заболевания человека. Галектин 9 — представитель класса галектинов типа «тандем-повтора». Наиболее высокий уровень экспрессии LGALS9 наблюдается в тканях толстого кишечника, легких, костном мозге, лимфоузлах, тимусе, печени, почках, эндокринных железах, плаценте, коже, гладких мышцах, жировой ткани.
У статті на підставі літературних джерел наведені статистичні дані середньорічних рівнів споживання лактози в країнах світового співтовариства, розглянуто клінічне значення надмірного вмісту лактози в дієті залежно від поліморфізму гена лактази. Лактоза — основне джерело енергії для дітей перших місяців життя, що забезпечує близько 40–45 % добової енергетичної потреби організму дитини. Порушення всмоктування лактози (мальабсорбція), при дефіциті ферменту лактази, супроводжується симптомами лактозної інтолерантності. Інтерес до вивчення імуномодулюючого впливу β-галактозиду лактози пов’язаний з інгібіцією нею галектину 9 (Gal-9), що регулює Т-клітинні імунні реакції за участю Т-хелперних клітин 1-го і 17-го типів (Тh1, Тh17) і Т-регуляторних лімфоцитів (Treg), що залучені до численних імуноопосередкованих захворювань людини. Галектин 9 — представник класу галектинів типу «тандем-повтору». Найбільш високий рівень експресії LGALS9 спостерігається в тканинах товстого кишечника, легенів, кісткового мозку, лімфовузлів, тимуса, печінки, нирок, ендокринних залоз, плаценти, шкіри, гладких м’язів, жирової тканини.
In the article on the basis of the literature there has been considered the statistics of average consumption of lactose in the countries of the world community, reviewed the clinical significance of the excess lactose in the diet depending on the polymorphism of the lactase gene. Lactose is the main source of energy for the children of the first months of life, which provides about 40–45 % of the daily energy needs of a body of a child. Lactose malabsorption, deficiency of the enzyme lactase is accompanied by symptoms of lactose intolerance. Interest in the study of the influence of an immunomodulatory β-galactoside lactose was caused by the suppression of its galectin 9 (Gal-9), the regulatory T-cell immune response involving T-helper cells 1 and 17 (Th1, Th17) and regulatory T-lymphocytes (Treg), which are involved in many immune-mediated human diseases. Galectin 9 is the representative of the class of galectins such as «tandem repeat». The highest level of LGALS9 expression is observed in the tissues of colon, lung, bone marrow, lymph nodes, thymus, liver, kidney, endocrine glands, placenta, skin, smooth muscle, adipose tissue.
лактоза, полиморфизмы LCT, галектин 9, воспаление, экзогенная лактаза.
лактоза, поліморфізми LCT, галектин 9, запалення, екзогенна лактаза.
lactose, LCT polymorphisms, galectin 9, inflammation, exogenous lactase.
Статья опубликована на с. 104-109
Введение
/106.jpg)
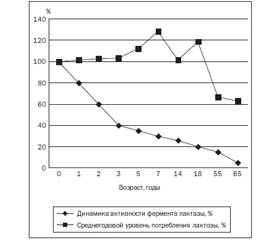
Характеристика фермента лактазы
Дериваты лактозы и их действие
Галектин 9
1. Абатуров А.Е. Роль лактазной недостаточности у детей / А.Е. Абатуров, А.А. Никулина, Л.Л. Петренко // Международный журнал педиатрии, акушерства и гинекологии. — 2015. — № 2(7). — С. 51-62.
2. Абатуров А.Е. Персистенция и недостаточность лактазы / А.Е. Абатуров, Ю.Ю. Степанова, А.А. Никулина // Педиатрия. Восточная Европа. — 2015. — № 3(11). — С. 31-43.
3. Гуліч М.П. Забезпеченість дітей кальцієм: роль молочних продуктів, шляхи корекції / М.П. Гуліч, Т.В. Поліщук // Еnvironment & health. — 2012. — № 4. — С. 61-65.
4. Делягин В.М. Полиморфизм гена лактазы у детей с атопическими заболеваниями / В.М. Делягин, К.Г. Каграманова, Е.Г. Шугурина, И.В. Сичинава, М.В. Соколова, С.А. Боринская и др. // Педиатрия. — 2008. — № 87(4). — С. 16-24.
5. Brüssow H. Nutrition, population growth and disease: a short history of lactose / H. Brüssow // Environ Microbiol. — 2013. — № 15. — Р. 2154-61. — Doi: 10.1111/1462-2920.12117.
6. Burger J. The lactase-persistence-associated allele in early Neolithic Europeans / J. Burger, M. Kirchner, B. Bramanti, W. Haak et al. // Proc. Natl. Acad. Sci. USA. — 2007 Mar 6. — № 104(10). — Р. 3736-41. — Doi: 10.1073/pnas.0607187104.
7. Campbell A.K. Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ transients and inhibit growth in E. coli / A.K. Campbell, R. Naseem, I.B. Holland, S.B. Matthews, K.T. Wann // Arch. Biochem. Biophys. — 2007. — № 468(1). — Р. 107-113. — Doi: 10.1016/j.abb.2007.09.006.
8. Cooper D.N.W. God must love galectines. He made so many of them / D.N.W. Cooper, S.H. Barondes // Glicobiology. — 1999. — № 9(10). — Р. 979-984. — PMID: 10521533.
9. Deng Y. Lactose Intolerance in Adults / Y. Deng, B. Misselwitz, N. Dai, M. Fox // Nutrients. — 2015 Sep 18. — № 7(9). — Р. 8020-35. — Doi: 10.3390/nu7095380.
10. Fang R. The homeodomain protein Cdx2 regulates lactase gene promoter activity during enterocyte differentiation / R. Fang // Gastroenterology. — 2000. — № 118(1). — Р. 115-127. — Doi: 10.1016/ S0016-5085(00)70420-3.
11. Harrington L.K. A reappraisal of lactose intolerance / L.K. Harrington, J.F. Mayberry // Int. J. Clin. Pract. — 2008. — № 62. — Р. 1541-1546. — Doi: 10.1111/j.1742-1241.2008.01834.x.
12. Heaney R.P. Dairy in take, dietary adequacy, and lactose intolerance / R.P. Heaney // Adv. Nutr. — 2013. — № 4(2). — Р. 151-156. — Doi: 10.3945/an.112.003368.
13. Ji J. Lactose intolerance and risk of lung, breast and ovarian cancers: aetiological clues from a population-based study in Sweden / J. Ji, J.K. Sundquist, N. Sundquist // Br. J. Cancer. — 2015, Jan 6. — № 112(1). — Р. 149-52. — Doi: 10.1038/bjc.2014.544.
14. Keryer-Bibens C. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9 / C. Keryer-Bibens, C. Pioche-Durieu, C. Villemant, S. Souquere et al. // BMC Cancer. — 2006. — № 6. — Р. 283. — Doi: 10.1186/1471-2407-6-283.
15. Lhuillier C. Impact of exogenous galectin-9 on human T cells: contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction / C. Lhuillier, C. Barjon, T. Niki, A. Gelin et al. // J. Biol. Chem. — 2015 May 6. — Pii: jbc.M115.661272.
16. Matsumoto R. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes / R. Matsumoto, H. Matsumoto, M. Seki, M. Hata // J. Biol. Chem. — 1998. — № 273. — Р. 16976-16984. — Doi: 10.1074/jbc.273.27.16976.
17. Mattar R. Lactose intolerance: diagnosis, genetic, and clinical factors / R. Mattar, D.F. Mazo, F.J. Carrilho // Clin. Exp. Gastroenterol. — 2012. — № 5. — Р. 113-21. — Doi: 10.2147/CEG.S32368.
18. Misselwitz B. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment / B. Misselwitz, D. Pohl, H. Frü–hauf, M. Fried et al. // United European Gastroenterol. J. — 2013. — № 1(3). — Р. 151-159. — Doi: 10.1177/2050640613484463.
19. Pereira P.C. Milk nutritional composition and its role in human healt / P.C. Pereira // Nutrition. — 2014. — № 30(6). — Р. 619-627. — Doi: 10.1016/j.nut.2013.10.011.
20. Prentice A.M. Dairy products in global public health / A.M. Prentice // Am. J. Clin. Nutr. — 2014 May. — № 99(5). — Р. 1212-1216. — Doi:10.3945/ajcn.113.073437.
21. Saita N. Association of galectin-9 with eosinophil apoptosis / N. Saita, E. Goto, T. Yamamoto, I. Cho et al. // Int. Arch. Allergy Immunol. — 2002. — № 128. — Р. 42-50. — Doi: 10.1159/000058002.
22. Savaiano D.A. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial / D.A. Savaiano, A.J. Ritter, T.R. Klaenhammer, G.M. James et al. // Nutr. J. — 2013. — № 160. — Р. 35. — Doi: 10.1186/1475-2891-12-160.
23. Sehrawat S. Influence of galectin-9/Tim-3 interaction on herpes simplex virus-1 latency / S. Sehrawat, P.B. Reddy, A. Suryawan–shi, N.K. Rajasagi et al. // J. Immunol. — 2011. — № 187(11). — Р. 5745-55. — Doi: 10.4049/jimmunol.1102105.
24. Sharon N. Lectins: Past, present and future // Biochem. Soc. Trans. — 2008. — № 36. — Р. 1457-1460. — Doi: 10.1042/BST0361457.
25. Smithson G. Selectin ligand expression and recruitment in inflammation, and together with fuc-tiv regulates naive T-cell trafficking to lymph nodes / G. Smithson, C.E. Rogers, P.L. Smith, E.P. Scheidegger // The Journal of Experimental Medicine. — 2001. — № 5. — Р. 601-614. — PMC219 5944.
26. Soriа С. Milk Consumption Per Capita Worldwide / C. Soriа // Food Beast: Map of Milk Consumption & Lactose Intole–rance Around the World. — 2015 Dec 29. — № 12. — Р. 345-364.
27. Suchy F.J. NIH consensus development conference statement: Lactose intolerance and health / F.J. Suchy, P.M. Brannon, T.O. Carpenter, J.R. Fernandez et al. // NIH Consens State Sci Statements. — 2010 Feb 24. — № 27(2). — Р. 1-27.
28. Than N.G. Galectins: Double-edged swords in the cross-roads of pregnancy complications and female reproductive tract inflammation and neoplasia / N.G. Than, R. Romero, A. Balogh, E. Karpati // J. Pathol. Transl. Med. — 2015. — № 49(3). — Р. 181-208. — Doi: 10.4132/jptm.2015.02.25.
29. Tureci O. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin’s disease / O. Tureci, H. Schmitt, N. Fadle, M. Pfreundschuh // J. Biol. Chem. — 1997. — № 272. — Р. 6416-6422. — Doi: 10.1074/jbc.272.10.6416.
30. Vladoiu M.C. Intracellular galectins in cancer cells: potential new targets for therapy (Review) / M.C. Vladoiu, M. Labrie, Y. St-Pierre // Int. J. Oncol. — 2014. — № 44(4). — Р. 1001-14. — Doi: 10.3892/ijo.2014.2267.
31. Wang Y. The lactase persistence/non-persistence polymorphism is controlled by a cis-acting element / Y. Wang, C.B. Harvey, W.S. Pratt, V.R. Sams et al. // Hum. Mol. Genet. — 1995. — № 4(4). — Р. 657-62. — Doi: 10.1093/hmg/4.4.657.
32. Wahlqvist M.L. Lactose nutrition in lactase nonpersi–sters / M.L. Wahlqvist // Asia Pac. J. Clin. Nutr. — 2015 Dec. — № 24(1). — Р. 21-5. — Doi: 10.6133/apjcn.2015.24.s1.04.
33. Wilt T.J. Lactose intolerance and health / T.J. Wilt, A. Shaukat, T. Shamliyan et al. // Evid. Rep. Technol. Assess (Full Rep). — 2010 Feb. — (192). — Р. 1-410. — PMID: 20629478.
34. Yoshida H. Interleukin-1beta stimulates galectin-9 expression in human astrocytes / H. Yoshida, T. Imaizumi, M. Kumagai, K. Kimura et al. // Neuroreport. — 2001. — № 12. — Р. 3755-3758. — Doi: 10.1371/journal. pone. 0009504.
35. Zheng X. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices / X. Zheng, H. Chu, Y. Cong, Y. Deng // Neurogastroenterol Motil. — 2015 Aug. — № 27(8). — Р. 1138-46. — Doi: 10.1111/nmo.12602.
1. Abaturov AA, Nikulina AA, Petrenko LL. [The role of lactase deficiency in children]. International Journal of Pediatrics, Obstetrics and Gynecology]. 2015; 2(7): 51-62. Russian.
2. Abaturov AA, Stepanova YuYu, Nikulina AA. [Persistence and lack of lactase]. Pediatrics. Eastern Europe. 2015; 3(11): 31-43. Russian.
3. Hulich MP, Polishchuk TV. [Availability calcium children: the role of dairy products, ways of correction]. Еnvironment & health. 2012; 4:61-65. Ukrainian.
4. Delyagin VM, Kagramanova KG, Shugurina EG. i dr. [Lactase gene polymorphism in children with atopic diseases]. Pediatrics. 2008; 87(4):16-24. Russian.
5. Brüssow H. Nutrition, population growth and disease: a short history of lactose. Environ Microbiol. 2013;15:2154–61. doi: 10.1111/1462-2920.12117.
6. Burger J, Kirchner M, Bramanti B et al. The lactase-persistence-associated allele in early Neolithic Europeans. Proc. Natl. Acad. Sci. USA. 2007; 104: 3736–3741. doi: 10.1073/pnas.0607187104.
7. Campbell AK, Naseem R, Holland IB et al. Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ transients and inhibit growth in E. coli. Arch. Biochem. Biophys.2007 Dec 1;468(1):107-113. doi: 10.1016/j.abb.2007.09.006.
8. Cooper DNW, Barondes SH. God must love galectines. He made so many of them. Glicobiology.1999; 9 (10):979–984. PMID: 10521533.
9. Deng Y, Misselwitz B, Dai N et al. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients. 2015;7(9):8020-35. doi: 10.3390/ nu70953804.
10. Fang R. The homeodomain protein Cdx2 regulates lactase gene promoter activity during enterocyte differentiation. Gastroenterology 2000; 118 ( 1): 115-127. doi:10.1016/S0016-5085(00)70420-3.
11. Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176(3):1411–20. doi: 10.4049/jimmunol.176.3.1411.
12. Heaney RP Dairy in take, dietary adequacy, and lactose intolerance. Adv. Nutr. 2013; 4(2):151-156. doi: 10.3945/an.112.003368.
13. Ji J, Sundquist JK, Sundquist N. Lactose intolerance and risk of lung, breast and ovarian cancers: aetiological clues from a population-based study in Sweden. Br J Cancer. 2015, Jan 6;112(1):149-52. doi: 10.1038/bjc.2014.544.
14. Keryer-Bibens C, Pioche-Durieu C, Villemant C et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006; 6: 283. doi: 10.1186/1471-2407-6-283.
15. Lhuillier C, Barjon C, Niki T et al. Impact of exogenous galectin-9 on human T cells: contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction. J. Biol. Chem. 2015; 290(27). doi:10.1074/jbc.M115.661272.
16. Matsumoto R, Matsumoto H, Seki M, Hata M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem 1998;273:16976-16984. . doi: 10.1074/jbc.273.27.16976.
17. Mattar R, Mazo DF. Intolerância à lactose: mudança de paradigmas coma biologia molecular. Rev. Assoc. Med. Bras. 2010; 56(2):230-236.doi.org/10.1590/S0104-42302010000200025.
18. Misselwitz B, Pohl D, Frühauf H . Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol. J. 2013; 1(3):151-159. doi: 10.1177/2050640613484463.
19. Pereira PC Milk nutritional composition and its role in human healt. Nutrition. 2014; 30(6):619-627. doi: 10.1016/j.nut.2013.10.011.
20. Prentice A.M. Dairy products in global public health. Am J Clin Nutr. 2014 May;99(5):1212-1216. doi:10.3945/ajcn.113.073437.
21. Saita N, Goto E, Yamamoto T et al. Association of galectin-9 with eosinophil apoptosis. Int Arch Allergy Immunol. 2002;128:42–50. doi:10.1159/000058002.
22. Savaiano DA, Ritter AJ, Klaenhammer TR, James GM, Longcore AT, Chandler JR et al. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial. Nutr. J. 2013; 12:160;35. doi: 10.1186/1475-2891-12-160.
23. Sehrawat S, Reddy PB, Suryawanshi A et al. Influence of galectin-9/Tim-3 interaction on herpes simplex virus-1 latency. J Immunol. 2011 Dec 1;187(11):5745-55. doi: 10.4049/ jimmunol.1102105.
24. Sharon N. Lectins: Past, present and future. Biochem Soc Trans. 2008; 36:1457–1460. doi:10.1042/BST0361457.
25. Smithson G, Rogers CE, Smith PL, Scheidegger EP. Selectin Ligand Expression and Recruitment in Inflammation, and Together with Fuc-TIV Regulates Naive T Cell Trafficking to Lymph Nodes. The Journal of Experimental Medicine. 2001;194: 601–614. PMC219 5944.
26. Soriа С. Milk Consumption Per Capita Worldwide. Food Beast: Map of Milk Consumption & Lactose Intolerance Around the World. 2015 Dec 29; 12:345-364.
27. Suchy FJ, Brannon PM, Carpenter TO et al. NIH consensus development conference statement: Lactose intolerance and health. NIH Consens State Sci Statements. 2010; 27(2):1-27// http://consensus.nih.gov.
28. Than NG, Romero R, Balogh A. et al. Galectins: Double-edged Swords in the Cross-roads of Pregnancy Complications and Female Reproductive Tract Inflammation and Neoplasia. J. Pathol. Transl. Med. 2015 May; 49(3):181-208. doi: 10.4132/jptm. 2015. 02.25. Epub 2015 May 1; http: //www.ncbi.nlm.nih.gov/gene.
29. Tureci O, Schmitt H, Fadle N, Pfreundschuh M, Sahin U. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin’s disease. J Biol Chem 1997;272:6416-6422. doi:10.1074/jbc.272.10.6416.
30. Vladoiu MC, Labrie M, St-Pierre Y. Intracellular galectins in cancer cells: potential new targets for therapy (Review). Int J Oncol. 2014;44(4):1001-14. doi: 10.3892/ijo. 2014.2267.
31. Wang Y, Harvey CB, Pratt WS et al. The lactase persistence/non-persistence polymorphism is controlled by a cis-acting element. Hum Mol Genet. 1995; 4(4):657-62.doi: 10.1093/hmg/4.4.657.
32. Wahlqvist ML Lactose nutrition in lactase nonpersisters. Asia Pac J Clin Nutr. 2015 Dec;24(1):21-5. doi: 10.6133/apjcn.2015.24.s1.04.
33. Wilt TJ, Shaukat A, Shamliyan T et al. Lactose intolerance and health. Evid Rep Technol Assess (Full Rep). 2010 Feb;(192):1-410. PMID:20629478.
34. Yoshida H, Imaizumi T, Kumagai M. et al. Interleukin-1beta stimulates galectin-9 expression in human astrocytes. Neuroreport. 2001;12:3755–3758. doi: 10.1371/journal. pone. 0009504.
35. Zheng X, Chu H, Cong Y et al. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: Prevalence, risk factors, and impact on food choices. Neurogastroenterol. Motil. 2015;27:1138–1146. doi: 10.1111/nmo.12602.

/105.jpg)