Журнал «Здоровье ребенка» 2 (70) 2016
Вернуться к номеру
Медикаментозное управление продукцией антимикробных пептидов при муковисцидозе у детей
Авторы: Абатуров А.Е., Бабич В.Л. - ГУ «Днепропетровская медицинская академия Министерства здравоохранения Украины», г. Днепропетровск, Украина
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
В статье представлены сведения о медикаментозном управлении продукцией антимикробных пептидов при муковисцидозе у детей. Приведены данные о типах и классах мутаций гена трансмембранного регуляторного протеина муковисцидоза (CFTR) и их ассоциации с клиническими особенностями кистозного фиброза. Представлено значение антимикробных пептидов в хроническом инфекционно-воспалительном процессе органов дыхания при муковисцидозе. Продемонстрированы молекулярные механизмы влияния витамина D на экспрессию антимикробных пептидов в респираторном тракте при муковисцидозе, а также проанализированы новые возможности применения витамина D в терапии детей с данным заболеванием.
У статті подані відомості про медикаментозне управління продукцією антимікробних пептидів при муковісцидозі у дітей. Наведено дані про типи та класи мутацій гена трансмембранного регуляторного протеїну муковісцидозу (CFTR) та їх асоціації з клінічними особливостями кістозного фіброзу. Наведено значення антимікробних пептидів у хронічному інфекційно-запальному процесі органів дихання при муковісцидозі. Продемонстровані молекулярні механізми впливу вітаміну D на експресію антимікробних пептидів у респіраторному тракті при муковісцидозі, а також проаналізовані нові можливості застосування вітаміну D у терапії дітей із даним захворюванням.
This article provides information about drug management of antimicrobial peptide production in cystic fibrosis in children. The data are provided on the types and classes of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, and their association with clinical features of cystic fibrosis. The value of antimicrobial peptides in chronic infectious-inflammatory process of the respiratory organs in cystic fibrosis is described. The molecular mechanisms of vitamin D effect on the expression of antimicrobial peptides in the respiratory tract in cystic fibrosis were shown, as well as new limitations of using vitamin D in the therapy of children with this disease were analyzed.
муковисцидоз, дети, антимикробные пептиды, витамин D.
муковісцидоз, діти, антимікробні пептиди, вітамін D.
cystic fibrosis, children, antimicrobial peptides, vitamin D.
Статья опубликована на с. 142-149
Актуальность
Эпидемиология муковисцидоза
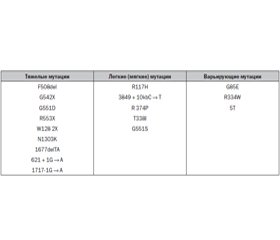
Ген CFTR
/143.jpg)
Заключение
1. Абатуров А.Е. Дефензины и дефензин-зависимые заболевания / А.Е. Абатуров, О.Н. Герасименко, И.Л. Высочина, Н.Ю. Завгородняя. — О.: Издательство ВМВ, 2011. — 265 с.
2. Амелина Е.Л. Ингаляционный тобрамицин в лечении хронической синегнойной инфекции при муковисцидозе / Е.Л. Амелина, С.А. Красовский // Пульмонология. — 2013. — № 4. — С. 109-114.
3. Бобровничий В.И. Современные подходы к диагностике и лечению синегнойной инфекции у больных муковисцидозом / В.И. Бобровничий // Медицинский журнал. — 2012. — № 1(39). — С. 4-9.
4. Гембицкая Т.Е. Фенотипические особенности и генетическая неоднородность больных при поздней манифестации и неклассическом течении муковисцидоза / Т.Е. Гембицкая, Т.Э. Иващенко, А.Г. Черменский, Ю.А. Насыхова // Пульмонология. — 2014. — № 1. — С. 66-70.
5. Капранов Н.И. Современная диагностика, терапия и социальная адаптация больных муковисцидозом в Российской Федерации / Н.И. Капранов // Педиатрия. — 2014. — Т. 93, № 4. — С. 6-10.
6. Леженко Г.О. Патогенетичне значення антимікробних пептидів у реалізації антибактеріального захисту у дітей, хворих на муковісцидоз / Г.О. Леженко, О.Є. Абатуров, О.Є. Пашкова // Здоровье ребенка. — 2013. — № 3(46).
7. Муковісцидоз в Україні: проблема, що потребує негайних дій // Современная педиатрия. — 2014. — № 3 — С. 23-27.
8. Симонова О.И. Решение проблемы хронической синегнойной инфекции у детей с муковисцидозом / [О.И. Симонова, Ю.В. Горинова, А.В. Лазарева и др.] // Вопросы современной педиатрии. — 2014. — Т. 13, № 1. — С. 66-73.
9. Феклин В.А. Микробный пейзаж дыхательных путей при муковисцидозе у детей / [В.А. Феклин, В.П. Кандыба, Е.Г. Колиушко и др.] // Вісник Вінницького національного медичного університету. — 2009. — № 13(1/2). — С. 342.
10. Чернуха М.Ю. Фенотипические и генотипические особенности штаммов Burkholderia cepacia complex, выделенных от больных муковисцидозом / [М.Ю. Чернуха, Л.Р. Аветисян, И.А. Шагинян и др.] // Педиатрия. — 2014. — Т. 93, № 4. — С. 24-29.
11. Albercht M.T. Binding of protegrin-1 to Pseudomonas aeruginosa and Burkholderia cepacia / M.T. Albercht, W. Wang, O. Sha–mova, R.I. Lehrer, N.L. Schiller // Respiratory Research. — 2002. — Vol. 3(1). — P. 18-29.
12. APD: the Antimicrobial Peptide Database. — http://aps.unmc.edu/AP/.
13. Belostotsky V. A single high dose of ergocalciferol can be used to boost 25-hydroxyvitamin D levels in children / V. Belostotsky, Z. Mughal // Pediatr. Nephrol. — 2009. — Vol. 24. — P. 625-626.
14. Bikle D.D. Vitamin D regulation of immune function / D.D. Bikle // Vitam. Horm. — 2011. — Vol. 86. — Р. 1-21. — Doi: 10.1016/B978-0-12-386960-9.00001-0.
15. Boyle M. Failure of high dose ergocalciferol to correct vitamin D deficiency in adults with cystic fibrosis / M. Boyle, M. Noschese, S. Watts [et al.] // Am. J. Respir. Crit. Care Med. — 2005. — Vol. 172. — P. 212. — Doi: 10.1164/rccm.200403-387OC.
16. Brandt T. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase / T. Brandt, S. Breitenstein, H. von der Hardt, B. Tummler // Thorax. — 1995. — Vol. 50. — P. 880-882. — PMID: 7570441.
17. Brogden K. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? / K. Brogden // Nat. Rev. Microbiol. — 2005. — Vol. 3. — P. 238-250. — PMID: 15703760.
18. Chesdachai S. Treatment of vitamin D deficiency in cystic fibrosis / S. Chesdachai, V. Tangpricha // The Journal of Steroid Biochemistry and Molecular Biology. — 2015 Sep 10. — Doi: 10.1016/j.jsbmb.2015.09.013.
19. Darling K.E.A. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized respiratory epithelial cells / K.E.A. Darling, A. Dewar, T.J. Evans // Cellular Microbiology. — June 2004. — Vol. 6, I. 6. — Р. 521-533. — Doi: 10.1111/j.1462-5822.2004.00380.x.
20. Diamond G. The Roles of Antimicrobial Peptides in Innate Host Defense / G. Diamond, N. Beckloff, A. Weinberg, K.O. Kisich // Curr. Pharm. — 2009. — Vol. 15(21). — P. 2377-2392. — PMCID: PMC2750833.
21. Diamond T. Annual intramuscular injection of megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data / T. Diamond, K. Ho, P. Rohl, M. Meerkin // Med. J. Aust. — 2005. — Vol. 183(1). — P. 10-12.
22. Donlan R.M. Biofilms: survival mechanisms of clinically relevant microorganisms / R.M. Donlan, J.W. Costerton // Clin. Microbiol. Rev. — 2002. — Vol. 15. — Р. 167-193. — PMID: 11932229.
23. European Cystic Fibrosis Patient Registry. Version 01.2014. Annual Data Report. Cystic Fibrosis Foundation. — http://www.cff.org.
24. Ferec C. Assessing the Disease-Liability of Mutations in CFTR / C. Ferec, Garry R. Cutting // Cold Spring Harb. Perspect. Med. — 2012. — Vol. 2(12). — P. 009480. — Doi: 10.1101/cshperspect.a009480.
25. Ferguson J.H. Vitamin D supplementation for cystic fibrosis / J.H. Ferguson, A.B. Chang // Cochrane Database Syst. Rev. — 2014. — Vol. 14(5). — CD007298. — Doi: 10.1002/14651858.CD007298.pub4.
26. Green D. Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate / D. Green, K. Carson, A. Leonard et al. // J. Pediatr. — 2008. — Vol. 153. — Р. 554-559. — PMID: 15992330.
27. Grossmanna R.E. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation / R.E. Grossmanna, S.M. Zughaierbc, M. Kumarid et al. // Dermato-Endocrinology. — 2012. — Vol. 4(2). — Р. 191-197. — Doi:10.4161/derm.20332.
28. Herr Ch. Vitamin D increases anti-microbial activity in human airway epithelial cells / Ch. Herr, J. Niederstraßer, R. Bals // Large Image / 22nd Annual Congress, Munich, Germany, 2014. — Vol. 44(58).
29. Hiemstra P.S. Antimicrobial peptides and innate lung defenses: Role in infectious and non-infectious lung diseases and therapeutic applications / P.S. Hiemstra, G.D. Amatngalim, A.M. van der Does, Ch. Taube // Chest. — 2015. — Doi:10.1378/chest.15-1353.
30. Kerem B. The molecular basis for disease variability in cystic fibrosis / B. Kerem, E. Kerem // Eur. J. Hum. Genet. — 1996. — Vol. 4. — Р. 65-73.
31. Knudsen P.K. Differences in prevalence and treatment of Pseudomonas aeruginosa in cystic fibrosis centres in Denmark, Norway and Sweden / P.K. Knudsen, H.V. Olesen, N. Hiniby et al. // Journal of Cystic Fibrosis. — 2009. — Vol. 8. — Р. 135-142.
32. Maurya N. Association of CFTR gene mutation with bronchial asthma / N. Maurya, S. Awasthi, P. Dixit // Indian J. Med. Res. — 2012. — Vol. 135(4). — P. 469-478. — PMID: 22664493.
33. Nakatsuji T. Antimicrobial peptides: Old molecules with new ideas / T. Nakatsuji, R.L. Gallo // J. Invest. Dermatol. — 2012. — Vol. 132. — Р. 887-895. — Doi: 10.1038/jid.2011.387.
34. Nash E.F. «Cepacia syndrome» associated with Burkholderia cepacia (genomovar I) infection with cystic fibrosis / E.F. Nash, A. Thomas, R. Whitmill et al. // Pediatr. Pulmonal. — 2011. — Vol. 46(5). — P. 512-514. — Doi: 10.1002/ppul.21404.
35. Norton L. Prevalence of Inadequate Vitamin D Status and Associated Factors in Children With Cystic Fibrosis / L. Norton, S. Page, M. Sheehan et al. // Nutr. Clin. Pract. — 2015. — Vol. 30(1). — P. 111-116. — Doi: 10.1177/0884533614562839.
36. Oceandy D. Gene complementation of airway epithelium in the cystic fibrosis mouse is necessary and sufficient to correct the pathogen clearance and inflammatory abnormalities / D. Oceandy, B.J. McMorran, S.N. Smith, R. Schreiber, K. Kunzelmann, E.W.F.W. Alton, D.A. Hume, B.J. Wainwright // Hum. Mol. Genet. — 2002. — Vol. 11(9). — Р. 1059-1067. — Doi: 10.1093/hmg/11.9.1059.
37. Patrick A.E. Alteration of CFTR transmembrane span integration by disease-causing mutations / A.E. Patrick, A.L. Karamyshev, L. Millen, P.J. Thomas // Mol. Biol. Cell. — 2011. — № 22(23). — Р. 4461-4471. — Doi: 10.1091/mbc.E11-05-0396.
38. Pattison S.H. Molecular detection of CF lung pathogens: Current status and future potential / S.H. Pattison, G.B. Rogers, M. Crockard, J.S. Elborn, M.M. Tunney // Journal of Cystic Fibrosis. — 2013. — № 12(3). — Р. 194-205. — Doi: 10.1016/j.jcf.2013.01.007.
39. Pier G.B. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung / G.B. Pier, M. Grout, T.S. Zaidi // Proc. Natl. Acad. Sci. USA. — 1997. — Vol. 94. — Р. 12088-12093.
40. Pier G.B. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections / Gerald B. Pier // The National Academy of Sciences of the United States of America. — 2000. — Vol. 97, № 16. — Р. 8822-8828. — Doi: 10.1073/pnas.97.16.8822.
41. Pompilio A. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients / A. Pompilio, M. Scocchi, S. Pomponia et al. // Peptides. — 2011. — Vol. 32(9). — P. 1807-1814. — Doi: 10.1016/j.peptides.2011.08.002.
42. Pranting M. Mechanism and fitness costs of PR-39 resistance in Salmonella enterica serovar Typhimurium LT2 / M. Pranting, A. Negrea, M. Rhen, D. Andersson // Antimicrob. Agents Chemother. — 2008. — Vol. 52(8). — P. 2734-2741. — Doi: 10.1128/AAC.00205-08.
43. Richards M.J. Nosocomial infections in combined medical-surgical intensive care units in the United States / M.J. Richards, J.R. Edwards, D.H. Culver et al. // Infect. Control. Hosp. Epidemiol. — 2000. — Vol. 21(8). — Р. 510-515. — PMID: 10968716.
44. Rowntree R.K. The Phenotypic Consequences of CFTR Mutations / R.K. Rowntree, A. Harris // Annals of Human Gene–tics. — 2003. — Vol. 67. — P. 471-485. — Doi: 10.1046/j.1469-1809.2003.00028.x.
45. Sermet-Gaudelus I. European cystic fibrosis bone mineralization guidelines / I. Sermet-Gaudelus, M.L. Bianchi, M. Garabedian et al. // J. Cyst. Fibros. — 2011. — Vol. 10. — P. S16-S23. — Doi: 10.1016/S1569-1993(11)60004-0.
46. Shepherd D. Single high-dose oral vitamin D3 (stoss) therapy — A solution to vitamin D deficiency in children with cystic fibrosis? / D. Shepherd, Y. Belessis, T. Katz [et al.] // Journal of Cystic Fibrosis. — 2013. — Vol. 12(2). — P. 177-182. — Doi:10.1016/j.jcf.2012.08.007.
47. Tangpricha V. An update on the screening, diagnosis, management and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the cystic fibrosis foundation / V. Tangpricha, A. Kelly, A. Stephenson et al. // J. Clin. Endocrinol. Metab. — 2012. — Vol. 97(4). — P. 1082-1093. — Doi: http://dx.doi.org/10.1210/jc.2011-3050/.
48. The CF mutation database 2014. — http://www.genet.sickkids.on.ca/cfitr
49. Vandamme P. Classification and identification of Burkholderia cepacia complex: Past, present and future / P. Vandamme, P. Dawyndt // Syst. Appl. Microbiol. — 2011. — Vol. 34(2). — P. 87-95. — Doi: 10.1016/j.syapm.2010.10.002.
50. Verkman A.S. CFTR Inhibitors / A.S. Verkman, D. Synder, L. Tradtrantip, J.R. Thiagarajah, M.O. Anderson // Curr. Pharm. Des. — 2013. — № 19. — Р. 3529-3541. — PMID: 23331030.
51. Voglis S. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs / S. Voglis, K. Quinn, E. Tullis, M. Liu [et al.] // Am. J. Respir. Crit. Care Med. — 2009. — Vol. 180, № 2. — Р. 159-166.
52. Wang T.-T. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression / –T.-T. Wang, F.P. Nestel, V. Bourdeau [et al.] // The Journal of Immunology. — 2004. — Vol. 173(5). — Р. 2909-2912. — Doi: 10.4049/jimmunol.173.5.2909.
53. Wold Health Organization — http://www.who.int/genomics/public/geneticdiseases/en/index2.html.
54. Yedery R.D. Augmentation of Cationic Antimicrobial Peptide Production with Histone Deacetylase Inhibitors as a Novel Epigenetic Therapy for Bacterial Infections / R.D. Yedery, A.E. Jerse // Antibiotics. — 2015. — Vol. 4(1). — Р. 44-61. — Doi:10.3390/antibiotics4010044.
1. Abaturov AE, Gerasimenko ON, Visochina IL, Zavgorodnyaya NJ. [Defensins and defensin-dependent diseases]. Оdessa: Izdatelstvo VMV; 2011. 265 p. Russian.
2. Amelina EL, Krasovsky SA. [Inhaled tobramycin in the treatment of chronic Pseudomonas aeruginosa infections in cystic fibrosis]. Рulmonology. 2013;4:109-114. Russian.
3. Bobrovnichy VI. [Current approaches to diagnosis and treatment of Pseudomonas infections in patients with cystic fibrosis]. Medical Journal. 2012;1(39):4-9. Russian.
4. Gembitskaya TE, Ivashchenko TE, Chermensky AG, Nasyhova Y. [Phenotypic features and genetic heterogeneity of patients with late onset and course of cystic fibrosis nonclassical]. Рulmonology. 2014;1:66-70. Russian.
5. Kapranov NI. [Modern diagnostics, therapy and social adaptation of patients with cystic fibrosis in the Russian Federation]. Pediatrics. 2014;93(4):6-10. Russian.
6. Lezhenko GА, Abaturov АЕ, Pashkovа АЕ. Pathogenetic values antimicrobial peptides have implementation of antibacterial protection in children with cystic fibrosis. Child Health. – 2013:3(46). Ukrainian.
7. Cystic fibrosis in Ukraine: a problem that needs immediate action // Modern pediatrics. 2014;3:23-27. Ukrainian.
8. Simonova OI, Gorinova Y, Lazareva AV et al. [Addressing the chronic Pseudomonas aeruginosa infection in children with cystic fibrosis]. Problems of modern pediatrics. 2014;13(1):66-73. Russian.
9. Feklin VA, Kandiba VP, Koliushko EG et al. [Microbial landscape of the airways in cystic fibrosis in children]. Bulletin of the Vinnitsa National Medical University. 2009;13(1/2):342. Russian.
10. Chernuha MY, Avetisyan LR, Shahinian IA et al. [Phenotypic and genotypic characteristics strains of Burkholderia cepacia complex, isolated from patients with cystic fibrosis]. Pediatrics. 2014;93(4):24-29. Russian.
11. Albercht MT, Wang W, Shamova O, Lehrer RI, Schiller NL. Binding of protegrin-1 to Pseudomonas aeruginosa and Burkholderia cepacia. Respiratory Research. 2002:3(1);18-29.
12. APD: the Antimicrobial Peptide Database. - http://aps.unmc.edu/AP/.
13. Belostotsky V., Mughal Z. A single high dose of ergocalciferol can be used to boost 25-hydroxyvitamin D levels in children. Pediatr. Nephrol. 2009;24:625–626.
14. Bikle D.D. Vitamin D regulation of immune function. Vitam. Horm. 2011;86:1-21. doi: 10.1016/B978-0-12-386960-9.00001-0.
15. Boyle M, Noschese M, Watts S et al. Failure of high dose ergocalciferol to correct vitamin D deficiency in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;172:212. doi: 10.1164/rccm.200403-387OC.
16. Brandt T, Breitenstein S, von der Hardt H, Tummler B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax. 1995;50:880-882. PMID: 7570441.
17. Brogden K. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238-250. PMID: 15703760.
18. Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. The Journal of Steroid Biochemistry and Molecular Biology. Sep 2015. pii: S0960-0760(15)30073-X. - doi: 10.1016/j.jsbmb.2015.09.013.
19. Darling KEA, Dewar A, Evans TJ. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized respiratory epithelial cells. Cellular Microbiology. June 2004;6(6):521–533. DOI: 10.1111/j.1462-5822.2004.00380.x
20. Diamond G, Beckloff N, Weinberg A, Kisich KO. The Roles of Antimicrobial Peptides in Innate Host Defense. Curr Pharm. 2009;15(21):2377–2392. PMCID: PMC2750833.
21. Diamond T, Ho K, Rohl P, Meerkin M. Annual intramuscular injection of megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Med J Aust 2005;183(1):10–12.
22. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms Clin Microbiol Rev. 2002;15:167-193. PMID: 11932229.
23. European Cystic Fibrosis Patient Registry. Version 01.2014. Annual Data Report. Cystic Fibrosis Foundation. - http://www.cff.org.
24. Ferec C, Cutting GR. Assessing the Disease-Liability of Mutations in CFTR. Cold Spring Harb Perspect Med. 2012;2(12):009480. doi: 10.1101/cshperspect.a009480.
25. Ferguson JH, Chang AB. Vitamin D supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2014;14(5):CD007298. doi: 10.1002/14651858.CD007298.pub4.
26. Green D, Carson K, Leonard A et al. Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate J Pediatr. 2008;554–559. PMID: 15992330.
27. Grossmanna RE, Zughaierbc SM, Kumarid M et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation. Dermato-Endocrinology. 2012;4(2):191-197. DOI:10.4161/derm.20332.
28. Herr Ch, Niederstraßer J, Bals R.Vitamin D increases anti-microbial activity in human airway epithelial cells. Large Image. 22nd Annual Congress, Munich, Germany 6–2014:44 (58).
29. Hiemstra PS, Amatngalim GD, van der Does AM, Taube Ch. Antimicrobial peptides and innate lung defenses: Role in infectious and non-infectious lung diseases and therapeutic applications. Chest. 2015. doi:10.1378/chest.15-1353.
30. Kerem B, Kerem E. The molecular basis for disease variability in cystic fibrosis. Eur J Hum Genet. 1996;4:65–73.
31. Knudsen PK, Olesen HV, Hiniby N et al. Differences in prevalence and treatment of Pseudomonas aeruginosa in cystic fibrosis centres in Denmark, Norway and Sweden. Journal of Cystic Fibrosis. 2009;8:135142.
32. Maurya N, Awasthi S, Dixit P. Association of CFTR gene mutation with bronchial asthma / N. Maurya, // Indian J. Med. Res. – 2012. – Vol. 135(4). – P. 469–478. - PMID: 22664493.
33. Nakatsuji T, Gallo RL. Antimicrobial peptides: Old molecules with new ideas. J Invest Dermatol. 2012;132:887–895. doi: 10.1038/jid.2011.387.
34. Nash EF, Thomas A, Whitmill R et al. «Cepacia syndrome» associated with Burkholderia cepacia (genomovar I) infection with cystic fibrosis. Pediatr Pulmonal. 2011;46(5):512–514. doi: 10.1002/ppul.21404.
35. Norton L, Page S, Sheehan M et al. Prevalence of Inadequate Vitamin D Status and Associated Factors in Children With Cystic Fibrosis. Nutr Clin Pract. 2015;30(1):111-116. doi: 10.1177/0884533614562839.
36. Oceandy D, McMorran BJ, Smith SN, Schreiber R, Kunzelmann K, Alton EWFW, Hume DA, Wainwright BJ. Gene complementation of airway epithelium in the cystic fibrosis mouse is necessary and sufficient to correct the pathogen clearance and inflammatory abnormalities. Hum. Mol. Genet. 2002;11(9):1059-1067. doi: 10.1093/hmg/11.9.1059.
37. Patrick AE, Karamyshev AL, Millen L, Thomas PJ. Alteration of CFTR transmembrane span integration by disease-causing mutations. Mol Biol Cell. 2011;22(23):4461-4471. doi: 10.1091/mbc.E11-05-0396.
38. Pattison SH, Rogers GB, Crockard M, Elborn JS, Tunney MM. Molecular detection of CF lung pathogens: Current status and future potential. Journal of Cystic Fibrosis. 2013;12(3):194-205. doi: 10.1016/j.jcf.2013.01.007.
39. Pier, GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl Acad. Sci. USA. 1997;94:12088–12093.
40. Pier GB. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. The National Academy of Sciences of the United States of America. 2000;97(16):8822–8828. doi: 10.1073/pnas.97.16.8822.
41. Pompilio A, Scocchi M, Pomponia S et al. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides. 2011;32(9):1807–1814. doi:10.1016/j.peptides.2011.08.002.
42. Pranting M, Negrea A, Rhen M, Andersson D. Mechanism and fitness costs of PR-39 resistance in Salmonella enterica serovar Typhimurium LT2. Antimicrob Agents Chemother. 2008;52(8);2734–2741. doi: 10.1128/AAC.00205-08.
43. Richards MJ, Edwards JR, Culver DH et al. Nosocomial infections in combined medical-surgical intensive care units in the United States Infect Control Hosp Epidemiol. 2000;21(8):510-515. PMID: 10968716.
44. Rowntree RK, Harris A. The Phenotypic Consequences of CFTR Mutations. Annals of Human Genetics. 2003;67:471-485. DOI: 10.1046/j.1469-1809.2003.00028.x.
45. Sermet-Gaudelus I., Bianchi ML, Garabedian M et al. European cystic fibrosis bone mineralization guidelines. J Cyst Fibros. 2011;10:S16–S23. doi: 10.1016/S1569-1993(11)60004-0.
46. Shepherd D, Belessis Y, Katz T et al. Single high-dose oral vitamin D3 (stoss) therapy — A solution to vitamin D deficiency in children with cystic fibrosis? Journal of Cystic Fibrosis. – 2013; 12 (2):177–182. doi:10.1016/j.jcf.2012.08.007.
47. Tangpricha V, Kelly A, Stephenson A et al. An update on the screening, diagnosis, management and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the cystic fibrosis foundation. J Clin Endocrinol Metab. 2012;97(4):1082–1093. DOI: http://dx.doi.org/10.1210/jc.2011-3050/
48. The CF mutation database 2014. - http://www.genet.sickkids.on.ca/cfitr.
49. Voglis S, Quinn K, Tullis E, Liu M et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am. J. Respir. Crit. Care Med. 2009:180(2);159-166.
50. Vandamme P, Dawyndt P. Classification and identification of Burkholderia cepacia complex: Past, present and future. Syst Appl Microbiol. 2011;34(2):87–95. doi: 10.1016/j.syapm.2010.10.002.
51. Verkman AS, Synder D, Tradtrantip L, Thiagarajah JR, Anderson MO. CFTR Inhibitors. Curr Pharm Des. 2013;19:3529–3541. PMID: 23331030.
52. Wang T-T, Nestel FP, Bourdeau V et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. The Journal of Immunology. 2004;173(5):2909-2912. doi: 10.4049/jimmunol.173.5.2909.
53. Wold Health Organization – http://www.who.int/genomics/public/geneticdiseases/en/index2.html.
54. Yedery RD, Jerse AE. Augmentation of Cationic Antimicrobial Peptide Production with Histone Deacetylase Inhibitors as a Novel Epigenetic Therapy for Bacterial Infections. Antibiotics. 2015;4(1):44-61. doi:10.3390/antibiotics4010044.

/144.jpg)