Журнал «Здоровье ребенка» 2 (70) 2016
Вернуться к номеру
Клиническое значение избыточного содержания лактозы в диете (часть 2)
Авторы: Абатуров А.Е., Никулина А.А. - ГУ «Днепропетровская медицинская академия Министерства здравоохранения Украины», г. Днепропетровск, Украина
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
В статье на основании литературных данных изложены представления о клиническом значении избыточного содержания лактозы в диете. Лактоза представляет собой специфический ингибитор β-галактозидсвязывающего протеина — галектина-9 (Gal-9), который регулирует внутриклеточный метаболизм (клеточный рост, воспаление, иммунный ответ, апоптоз). Лактоза, конкурентно связываясь с Gal-9, предупреждает активацию Gal-9/Тim-3-ассоциированных сигнальных путей, что способствует пролиферации Т-хелперных клеток 1-го и 17-го типа, обусловливающих индукцию воспалительного процесса. Избыток лактозы способствует уменьшению представительства Treg-клеток, которые обладают иммуносупрессивным действием, и повышает инсулинорезистентность. Лактоза ингибирует взаимодействие Gal-9 с иммуноглобулином E и гиалуронсвязывающей молекулой СD44 и способствует аллергическим проявлениям. Приведены возможности использования препаратов экзогенной лактазы для патогномоничного лечения воспалительных и аллергических заболеваний у детей с лактазной недостаточностью.
В статті на підставі літературних даних викладені уявлення про клінічне значення надмірного вмісту лактози в дієті. Лактоза є інгібітором β-галактозидзв’язуючого протеїну — галектину-9 (Gal-9), який регулює внутрішньоклітинний метаболізм (клітинний ріст, запалення, імунну відповідь, апоптоз). Лактоза, конкурентно зв’язуючись з Gal-9, скасовує запуск сигнального механізму Gal-9/Tim-3 і посилює проліферацію Т-хелперних клітин 1-го та 17-го типу. Це призводить до загострення запальних процесів. Надлишок лактози сприяє зменшенню представництва Treg-клітин, що володіють імуносупресивною дією, і підвищує інсулінорезистентність. Лактоза інгібує взаємодію Gal-9 з імуноглобуліном Е та гіалуронзв’язуючою молекулою CD44 і сприяє алергічним проявам. Наведено можливості використання препаратів екзогенної лактази для патогномонічного лікування запальних та алергічних захворювань у дітей з лактазною недостатністю.
The article on the basis of published data presents the ideas about the clinical significance of excess lactose in the diet. Lactose is a specific inhibitor of β-galactoside-binding protein — galectin-9 (Gal-9) which regulates the intracellular metabolism (cell growth, inflammation, immune response, apoptosis). Lactose, competitively binding to Gal-9, prevents activation of Gal-9/TIM-3-associated signaling pathways that promotes proliferation of the T-helper 1 and 17 cells, causing the induction of inflammation. Excess lactose reduces Treg-cells representation, which have immunosuppressive action, and increases insulin resistance. Lactose inhibits the interaction of Gal-9 with immunoglobulin E and hyaluronan-binding molecule CD44 and contributes to allergic manifestations. The limitations of using exogenous lactase preparations for pathognomonic treatment of inflammatory and allergic diseases in children with lactase deficiency are presented.
лактоза, полиморфизмы LCT, галектин-9, воспаление, аллергия, экзогенная лактаза.
лактоза, поліморфізми LCT, галектин-9, запалення, алергія, екзогенна лактаза.
lactose, LCT polymorphisms, galectin-9, inflammation, allergy, exogenous lactase.
Статья опубликована на с. 150-157
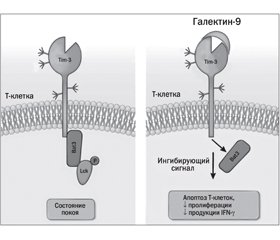
Биологическое действие галектина-9
/151.jpg)
Ингибирование галектина-9 лактозой как фактор, способствующий развитию хронических заболеваний
Инсулинорезистентность
Значение для развития ожирения избытка лактозы в диете
Возможности применения препаратов экзогенной лактазы
Заключение
1. Абатуров А.Е. Введение в иммунологию инфекционного процесса для педиатров и врачей общей практики — семейной медицины / А.Е. Абатуров, Е.А. Агафонова, О.Н. Герасименко, Е.Л. Кривуша. — К.: Джулия Принт, 2012. — 172.
2. Абатуров А.Е. Роль лактазной недостаточности у детей / А.Е. Абатуров, А.А. Никулина, Л.Л. Петренко // Международный журнал педиатрии, акушерства и гинекологии. 2015; 2 (7): 51-62.
3. Делягин В.М. Полиморфизм гена лактазы у детей с атопическими заболеваниями / В.М. Делягин, К.Г. Каграманова, Е.Г. Шугурина, И.В. Сичинава, М.В. Соколова, С.А. Боринская и др. // Педиатрия. 2008; 87 (4): 16-24.
4. Barjon C. A novel monoclonal antibody for detection of galectin-9 in tissue sections: application to human tissues infected by oncogenic viruses / C. Barjon, T. Niki, В. Verillaud et al. // Infectious agents and cancer. 2012; 7: 16. doi: 10.1186/1750-9378-7-16.
5. Chen X. Intestinal epithelial cells express galectin-9 in patients with food allergy that plays a critical role in sustaining allergic status in mouse intestine/ X. Chen, C.H. Song, Z.Q. Liu, B.S. Feng et al. // Allergy. 2011; 66: 1038-1046. doi: 10.1111/j.1398-9995.2011.02585.x.
6. Chou F.C. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice / F.C. Chou, S.J. Shieh, H.K. Sytwu // Eur. J. Immunol. 2009; 39 (9): 2403-2511. doi: 10.1002/eji. 200839177.
7. Cox L.M. Pathways in microbe-induced obesity/ L.M. Cox, J.M. Blaser // Cell Metab. 2013; 17 (6): 883-894. doi: 10.1016/j.cmet.2013. 05.004.
8. Enattah N. Identification of a variant associated with adult type hypolactasia/ N. Enattah, T. Sahi, E. Savilahti et al. // Nat. Genetic. 2002; 30: 233-237. doi: 10.1038/ng826.
9. Geng H. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response / H. Geng, G.M. Zhang, D. Li, H. Zhang et al. // J. Immunol. 2006; 176 (3): 1411-20. doi: 10.4049/jimmunol.176.3.1411.
10. Golden-Mason L. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells / L. Golden-Mason, B.E. Palmer, N. Kassam et al. // J. Virol. 2009 Sep; 83 (18): 9122-30. doi: 10.1128/JVI.00639-09.
11. Gorman J.V. Regulation of T cell responses by the receptor molecule Tim-3 / J.V. Gorman, J.D. Colgan // Immunol. Res. 2014; 59 (1–3): 56-65. doi: 10.1007/s12026-014-8524-1
12. Gumperz J.E. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining / J.E. Gumperz, S. Miyake, T. Yamamura, M.B. Brenner // J. Exp. Med. 2002 Mar 4; 195 (5): 625-36. doi: 10.1084/jem.20011786.
13. Haining W.N. Thinking inside the box: how T cell inhibitory receptors signa / W.N. Haining // Nat. Med. 2012 Sep; 18 (9): 1338-9. doi: 10.1038/nm.2921.
14. Han G. Tim-3: an activation marker and activation limiter of innate immune cells / G. Han, G. Chen, B. Shen, Y. Li // Front Immunol. 2013 Dec 10; 4: 449. doi: 10.3389/fimmu.2013.00449.
15. Hassan H.Y. Genetic diversity of lactase persistence in East African populations / H.Y. Hassan, A. van Erp, M. Jaeger, H. Tahir // BMC Res Notes. 2016, Jan 4; 9 (1): 8. doi: 10.1186/s13104-015-1833-1.
16. Jacobs J. Immune Checkpoint Modulation in Colorectal Cancer: What's New and What to Expect / J. Jacobs, E. Smits, F. Lardon, P. Pauwels, V. Deschoolmeester // J. Immunol. Res. 2015; 2015: 158038. doi: 10.1155/2015/158038.
17. Keryer-Bibens C. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9 / C. Keryer-Bibens, C. Pioche-Durieu, C. Villemant, S. Souquere et al. // BMC Cancer. 2006; 6: 283. doi: 10.1186/1471-2407-6-283
18. de Kivit S. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans / S. de Kivit, E. Saeland, A.D. Kraneveld, H.J.G. van de Kant et al. // Allergy. 2012; 67: 343-352. doi: 10.1111/j.1398-9995.2011.02771.x.
19. Koguchi K. Dysregulated T cell expression of TIM3 in multiple sclerosis / K. Koguchi, D.E. Anderson, L. Yang et al. // J. Exp. Med. 2006 Jun 12; 203 (6): 1413-8. doi: 10.1084/jem.20060210.
20. Kurose Y. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease / Y. Kurose, J. Wada, M. Kanzaki et al. // BMC Nephrol. 2013 Jan 22; 14: 23. doi: 10.1186/1471-2369-14-23.
21. Lee J. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis / J. Lee, J.M. Oh, J.W. Hwang, J.K. Ahn et al. // Scand. J. Rheumatol. 2011; 40 (5): 334-40. doi: 10.3109/03009742.2010.547871.
22. Leitner J. TIM-3 Does Not Act as a Receptor for Galectin-9 / J. Leitner, A. Rieger, W.F. Pickl, G. Zlabinger // PLOS. 2013 March 21. doi: 10.1371/journal.ppat.100325.
23. Lhuillier C. Impact of exogenous galectin-9 on human T cells: contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction / C. Lhuillier, C. Barjon, T. Niki, A. Gelin et al. // J. Biol. Chem. 2015 May 6; pii: jbc.M115.661272.
24. Li X. Involvement of T cell Ig Mucin-3 (Tim-3) in the negative regulation of inflammatory bowel disease / X. Li, G. Chen, Y. Li et al. // Clin. Immunol. 2010 Feb; 134 (2): 169-77. doi: 10.1016/j.clim.2009.09.012.
25. Li Y. The N- and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity / J. Feng, S. Geng, H. Wei et al. // Mol. Immunol. 2011; 48: 670-677. doi: 10.1016/j. molimm. 2010.11.011
26. Liberal R. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway / R. Liberal, C.R. Grant, B.S. Holder et al. // Hepatology. 2012 Aug; 56 (2): 677-86. doi: 10.1002/hep.25682.
27. Ma C.J. Cis-Association of Galectin-9 with Tim-3 Differentially Regulates IL-12/IL-23 Expressions in Monocytes via TLR Signa–ling / C.J. Ma, G.Y. Li, Y.Q. Cheng et al. // PLoS One. 2013, Aug 14; 8 (8): e72488. doi: 10.1371/journal.pone.0072488. eCollection 2013.
28. Madireddi S. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies / S. Madireddi, S.Y. Eun, S.W. Lee, I. Nemčovičová // J. Exp. Med. 2014; 211 (7): 1433-48. doi: 10.1084/jem.20132687.
29. Matsumoto R. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes / R. Matsumoto, H. Matsumoto, M. Seki, M. Hata // J. Biol. Chem. 1998; 273: 16976-16984. doi: 10.1074/jbc.273.27.16976.
30. Mitra S. Histological changes in intestine in semichronic diarrhea induced lactose enriched diet in rats: effect of Diarex Vet / S. Mitra, S. Ashisha, V. Udupa, S. Sheshadri // Ind. J. of Еxp. Вiol. 2003; 41: 21-215. PMID: 15267149.
31. Mrizak D. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells / D. Mrizak, N. Martin, C. Barjon, A.S. Jimenez-Pailhes et al. // J. Natl. Cancer. Inst. 2015; 107: 363. doi: 10.1093/jnci/dju363.
32. Niki T. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation / T. Niki, S. Tsutsui, S. Hirose, S. Aradono et al. // J. Biol. Chem. 2009; 284: 32344-32352. doi: 10.1074/jbc.M109.035196.
33. Norling L.V. Endogenous galectins and the control of the host inflammatory response / L.V. Norling, M. Perretti, D. Cooper // J. Endocrinol. 2009; 201 (2): 169-84. doi: 10.1677/JOE-08-0512.
34. Oomizu S. Cell surface galectin-9 expressing th cells regulate th17 and foxp3 (+) treg development by galectin-9 secretion / S. Oomizu, T. Arikawa, T. Niki, T. Kadowaki // PloS One. 2012; 7: e48574. doi: 10.1371/journal.pone.0048574.
35. Paasela M. Lactose inhibits regulatory T-cell-mediated suppression of effector T-cell interferon-γ and IL-17 production / M. Paasela, K.-L. Kolho, O. Vaarala et al. // Br. J. Nutr. 2014; 112 (11): 1819-1825. doi: 10.1017/S0007114514001998.
36. Rahman A.N. TIM-3 and Its Immunoregulatory Role in HIV Infection / A.N. Rahman, K. Clayton, S. Mujib, I.W. Fong, M.A. Ostrowski // J. Clin. Cell Immunol. 2012; 7: 007. doi: 10.4172/2155-9899.S7-007.
37. Raithel M. The malabsorption of commonly occurring mono and disaccharides: levels of investigation and differential diagnoses / M. Raithel, M. Weidenhiller, A.F. Hagel, U. Hetterich et al. // DtschArztebl. Int. 2013; 110 (46): 775-782. doi: 10.3238/arztebl.2013.0775.
38. Sakuishi K. Emerging Tim-3 functions in antimicrobial and tumor immunity / K. Sakuishi, P. Jayaraman, S.M. Behar, A.C. Anderson et al. // Trends Immunol. 2011 Aug; 32 (8): 345-9. doi: 10.1016/j.it.2011.05.003.
39. Sakaguchi S. Foxo1 and Foxo3 help Foxp3 / S. Sakaguchi, N. Ohkura // Immunity. 2010; 33 (6): 835-7. doi: 10.1016/j. immuni.2010.12.004.
40. Sehrawat S. Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8+T Cell Response/ S. Sehrawat, P.B.J. Reddy, N. Rajasagi, A. Suryawanshi et al. // PLoS Pathog. 2010; 6 (5): e1000882. doi: 10.1371/journal. ppat.1000882.
41. Shevach E.M. tTregs, pTregs, and iTregs: similarities and differences / E.M. Shevach, A.M. Thornton // Immunol Rev. 2014; 259 (1): 88-102. doi: 10.1111/imr.12160.
42. Su E.W. Galectin-9 regulates T helper cell function independently of Tim-3 / E.W. Su, S. Bi, L.P. Kane // Glycobiology. 2011; 21: 1258-1265. doi: 10.1093/glycob/cwq214.
43. Sziksz E. Galectin-9: a suppressor of food allergy? / E. Sziksz, A. Vannay, A. Haczku // Allergy. 2012; 67 (3): 293-5. doi: 10.1111/j.1398-9995. 2011. 02774.x.
44. Tang Z.H. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease / Z.H. Tang, S. Liang, J. Potter et al. // J. Immunol. 2013 Feb 15; 190 (4): 1788-96. doi: 10.4049/jimmunol.1202814.
45. Vignali D.A. How regulatory T cells work / D.A. Vignali, L.W. Collison, C.J. Workman // Nat. Rev. Immunol. 2008; 8 (7): 523-32. doi: 10.1038/nri2343.
46. de Vrese M. A combination of acid lactase from Aspergillus oryzae and yogurt bacteria improves lactose digestion in lactose maldigesters synergistically: A randomized, controlled, double-blind cross-over trial / M. de Vrese, C. Laue, B. Offick, E. Soeth, F. Repenning et al. // Clin. Nutr. 2015 Jun; 34 (3): 394-9. doi: 10.1016/j.clnu.2014.06.012.
47. Wiersma V.R. Therapeutic potential of Galectin-9 in human disease / V.R. Wiersma, M. de Bruyn, W. Helfrich, E. Bremer // Med. Res. Rev. 2013; 33: 102-126. doi: 10.1002/med.20249.
48. Wu C. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells // Immunity. 2014; 41 (2): 270-82. doi: 10.1016/j.immuni. 2014.06.011.
49. Yoshida H. Interleukin-1beta stimulates galectin-9 expression in human astrocytes / H. Yoshida, T. Imaizumi, M. Kumagai, K. Kimura et al. // Neuroreport. 2001; 12: 3755-3758. doi: 10.1371/journal. pone. 0009504.
50. Zhao Q. High level production of β-galactosidase exhibiting excellent milk-lactose degradation ability from Aspergillus oryzae by codon and fermentation optimization / Q. Zhao, F. Liu, Z. Hou, C. Yuan, X. Zhu // Appl Biochem Biotechnol. 2014 Mar; 172 (6): 2787-99. doi: 10.1007/s12010-013-0684-2.
51. Zhu C. (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity / C. Zhu, A.C. Anderson, A. Schubart, H. Xiong et al. // Nat. Immunol. 2005; 6: 1245-1252. doi: 10.1038/ni1271.
1. Abaturov AE, Agafonova A E, Gerasimenko ON, Krivusha EL. [Introduction to the immunology of infectious process for teachers and general practitioners - family medicine]. Kiev: OOO «Dzhuliya Print»; 2012; 172 р. Russian.
2. Abaturov AA, Nikulina AA, Petrenko LL. [The role of lactase deficiency in children]. International Journal of Pediatrics, Obstetrics and Gynecology]. 2015; 2(7): 51-62. Russian.
3. Delyagin VM, Kagramanova KG, Shugurina EG. i dr. [Lactase gene polymorphism in children with atopic diseases]. Pediatrics. 2008; 87(4):16-24. Russian.
4. Barjon C, Niki T, Verillaud B et al. A novel monoclonal antibody for detection of galectin-9 in tissue sections: application to human tissues infected by oncogenic viruses. Infectious agents and cancer. 2012;7: 16. doi:10.1186/1750-9378-7-16. doi:10.1186/1750-9378-7-16.
5. Chen X, Song CH, Liu ZQ et al. Intestinal epithelial cells express galectin-9 in patients with food allergy that plays a critical role in sustaining allergic status in mouse intestine. Allergy.2011;66:1038–1046. doi: 10.1111/j.1398-9995.2011.02585.x.
6. Chou F.C., Shieh S.J., Sytwu H.K. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur. J. Immunol. 2009; 39(9): 2403-2511. doi: 10.1002/eji. 200839177. doi: 10.1002/eji. 200839177.
7. Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab. 2013; 17(6): 883–894. doi:10.1016/j.cmet. 2013. 05.004. doi:10.1016/j.cmet.2013. 05.004.
8. Enattah N, Sahi T, Savilahti E et al. Identification of a variant associated with adult type hypolactasia. Nat. Genetic. 2002; 30:233–237. doi:10.1038/ng826.
9. Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176(3):1411–20. doi: 10.4049/jimmunol.176.3.1411.
10. Golden-Mason L, Palmer BE, Kassam N et al Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009 Sep;83(18):9122-30. doi: 10.1128/JVI.00639-09.
11. Gorman JV, Colgan JD. Regulation of T- cell responses by the receptor molecule Tim-3. Immunol Res. 2014; 59(1-3):56-65. doi:10.1007/s12026-014-8524-1
12. Gumperz JE, Miyake S, Yamamura Т, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002 Mar 4;195(5):625-36. doi: 10.1084/jem.20011786.
13. Haining WN. Thinking inside the box: how T cell inhibitory receptors signal. Nat Med. 2012 Sep;18(9):1338-9. doi: 10.1038/nm.2921.
14. Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013 Dec 10; 4:449. doi: 10.3389/fimmu. 2013.00449.
15. Hassan HY, van Erp A., Jaeger M., Tahir H. Genetic diversity of lactase persistence in East African populations. BMC Res Notes. 2016, Jan 4;9(1):8. doi: 10.1186/s13104-015-1833-1.
16. Jacobs J, Smits E, Lardon F, Pauwels P, Deschoolmeester V. Immune Checkpoint Modulation in Colorectal Cancer: What's New and What to Expect. J Immunol Res. 2015; 2015:158038. doi: 10.1155/2015/158038.
17. Keryer-Bibens C, Pioche-Durieu C, Villemant C et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006; 6: 283. doi: 10.1186/1471-2407-6-283.
18. de Kivit S, Saeland E, Kraneveld AD et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy.2012;67:343–352. doi: 10.1111/j.1398-9995.2011.02771.x.
19. Koguchi K, Anderson DE, Yang L et al. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006 Jun 12;203(6):1413-8. doi: 10.1084/jem.20060210.
20. Kurose Y, Wada J, Kanzaki M et al. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease. BMC Nephrol. 2013 Jan 22;14:23. doi: 10.1186/1471-2369-14-23.
21. Lee J, Oh JM, Hwang JW et al. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis.Scand J Rheumatol. 2011;40(5):334–40. doi: 10.3109/03009742.2010.547871.
22. Leitner J, Rieger А, Pickl WJ et al. TIM-3 Does Not Act as a Receptor for Galectin-9. PLOS. 2013; 9(3):21 doi: 10.1371/journal.ppat.100325.
23. Lhuillier C, Barjon C, Niki T et al. Impact of exogenous galectin-9 on human T cells: contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction. J. Biol. Chem. 2015; 290(27). doi:10.1074/jbc.M115.661272.
24. Li X, Chen G, Li Y et al. Involvement of T cell Ig Mucin-3 (Tim-3) in the negative regulation of inflammatory bowel disease. Clin Immunol. 2010 Feb;134(2):169 -77. doi: 10.1016/j.clim.2009.09.012.
25. Li Y, Feng J, Geng S et al. The N- and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity. Mol Immunol. 2011; 48:670–677. doi: 10.1016/j. molimm. 2010.11.011.
26. Liberal R, Grant CR, Holder BS et al. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology. 2012 Aug; 56(2):677-86. doi: 10.1002/hep.25682.
27. Ma CJ, Li GY, Cheng YQ et al. Cis-Association of Galectin-9 with Tim-3 Differentially Regulates IL-12/IL-23 Expressions in Monocytes via TLR Signaling. PLoS One. 2013 Aug 14;8(8):e72488. doi: 10.1371/journal.pone.0072488. eCollection 2013.
28. Madireddi S. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies/ S. Madireddi, S.Y. Eun, S.W. Lee, I. Nemčovičová// J Exp Med. 2014;211(7):1433-48. doi: 10.1084/jem.20132687.
29. Matsumoto R, Matsumoto H, Seki M, Hata M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem 1998;273:16976-16984. . doi: 10.1074/jbc.273.27.16976.
30. Mitra S, Ashisha S, Udupa V, Sheshadri S. Histological changes in intestine in semichronic diarrhea induced lactose enriched diet in rats: effect of Diarex Vet. Ind. J. of Еxp. Вiol. 2003; 41: 21–215. PMID:15267149.
31. Mrizak D, Martin N, Barjon C et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015:107, 363. doi: 10.1093/jnci/dju363.
32. Niki T, Tsutsui S, Hirose S. et al. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation. J Biol Chem. 2009; 284: 32344–32352. doi: 10.1074/jbc.M109.035196.
33. Norling LV, Perretti M, Cooper D. Endogenous galectins and the control of the host inflammatory response. J. Endocrinol. 2009; 201(2):169-84. doi: 10.1677/JOE-08-0512
34. Oomizu S, Arikawa T, Niki T et al. Cell surface galectin-9 expressing th cells regulate Th17 and Foxp3(+) Treg development by galectin-9 secretion. PloS One 7, e48574. doi: 10.1371/journal.pone.0048574.
35. Paasela M, Kolho K-L, Vaarala O. et al. Lactose inhibits regulatory T-cell-mediated suppression of effector T-cell interferon-γ and IL-17 production. Br. J. Nutr. 2014; 112 (11):P1819–1825. doi:10.1017/S0007114514001998.
36. Rahman AN, Clayton K, Mujib S, Fong IW, Ostrowski MA. TIM-3 and Its Immunoregulatory Role in HIV Infection. J Clin Cell Immunol. 2012;7:007. doi:10.4172/2155-9899.S7-007.
37. Raithel M, Weidenhiller M, Hagel AF. The malabsorption of commonly occurring mono and disaccharides: levels of investigation and differential diagnoses. DtschArztebl. Int. 2013; 110(46):775-782. doi: 10.3238/ arztebl.2013.0775.
38. Sakaguchi S, Ohkura N. Foxo1 and Foxo3 help Foxp3. mmunity. 2010;33(6):835-7. doi: 10.1016/j.immuni.2010.12.004.
39. Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011; 32(8):345-9. doi: 10.1016/j.it. 2011. 05.003.
40. Sehrawat S, Reddy PBJ, Rajasagi N et al. Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8+T Cell Response. PLoS Pathog. 2010; 6(5): e1000882. doi:10.1371/journal. ppat.1000882.
41. Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014 ;259(1):88-102. doi: 10.1111/imr.12160.
42. Su EW, Bi S, Kane LP. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology. 2011; 21:1258–1265. doi: 10.1093/glycob/cwq214.
43. Sziksz E, Vannay A, Haczku A. Galectin-9: a suppressor of food allergy? Allergy. 2012; 67(3):293-5. doi: 10.1111/j.1398-9995. 2011. 02774.x.
44. Than NG, Romero R., Balogh A. et al. Galectins: Double-edged Swords in the Cross-roads of Pregnancy Complications and Female Reproductive Tract Inflammation and Neoplasia. J. Pathol. Transl. Med. 2015 May; 49(3):181-208. doi: 10.4132/jptm. 2015. 02.25. Epub 2015 May 1; http: //www.ncbi.nlm.nih.gov/gene.
45. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523-32. doi: 10.1038/nri2343.
46. de Vrese M, Laue C, Offick B, Soeth E, Repenning F et al. A combination of acid lactase from Aspergillus oryzae and yogurt bacteria improves lactose digestion in lactose maldigesters synergistically: A randomized, controlled, double-blind cross-over trial. Clin Nutr. 2015 Jun;34(3):394-9. doi: 10.1016/j.clnu.2014.06.012.
47. Wiersma VR, de Bruyn M, Helfrich W et al. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2013;33: 102–126. doi: 10.1002/med.20249.
48. Wu C. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41(2):270-82. doi: 10.1016/j.immuni. 2014.06.011.
49. Yoshida H, Imaizumi T, Kumagai M. et al. Interleukin-1beta stimulates galectin-9 expression in human astrocytes. Neuroreport. 2001;12:3755–3758. doi: 10.1371/journal. pone. 0009504
50. Zhao Q, Liu F, Hou Z, Yuan C, Zhu X. High level production of β-galactosidase exhibiting excellent milk-lactose degradation ability from Aspergillus oryzae by codon and fermentation optimization. Appl Biochem Biotechnol. 2014 Mar;172(6):2787-99. doi: 10.1007/s12010-013-0684-2.
51. Zhu C, Anderson AC, Schubart A et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005; 6: 1245-1252. doi:10.1038/ni1271.

/152.jpg)
/151_2.jpg)