Журнал «Боль. Суставы. Позвоночник» 1 (21) 2016
Вернуться к номеру
Pitfalls in the Development of Drugs in Sarcopenia
Авторы: Reginster J.-Y., Bruyère O. - Department of Public Health, Epidemiology and Health Economics, University of Liège, Liège, Belgium
Рубрики: Ревматология, Травматология и ортопедия
Разделы: Медицинские форумы
Версия для печати
The article was published on p. 82
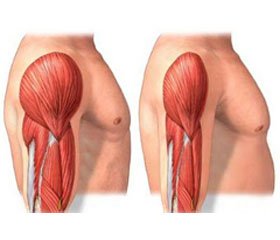
Sarcopenia is characterized by a loss of muscle mass and muscle function. Sarcopenia is frequently a precursor of frailty, mobility, disability and premature death. It has a high prevalence in older population and therefore presents a considerable social and economic burden. Potential treatments are under development but there are no guidelines to support regulatory studies for new chemical entities. There are numerous gaps in our knowledge, particularly concerning risk assessment. It would be instructive to build a risk model similar to the one for osteoporosis (FRAX). The value of indexing threshold values for sarcopenia measures and outcomes needs to be further investigated, using a risk-based analysis, for one of the strong clinical endpoints. A consensus core outcome set would bring standardization and comparability to research in sarcopenia and therefore, would help improve the evidence base for health care. A common operational definition of sarcopenia is urgently needed. It is time to address the lack of regulatory guidelines concerning the evaluation of drugs for the prevention and treatment of sarcopenia.