Резюме
Проведено комплексне обстеження 204 дітей віком від 2 місяців до 3 років із позалікарняною пневмонією. Установлено, що в дітей раннього віку, хворих на позалікарняну пневмонію, провідним етіологічним фактором є бактерії Streptococcus pneumoniae (36,8 %). Вміст ендогенних антимікробних пептидів визначено в сироватці крові 20 дітей раннього віку, хворих на позалікарняну пневмонію, та 17 дітей групи контролю. Доведено, що розвиток позалікарняної пневмонії в дітей раннього віку відбувається на фоні зниження вмісту в сироватці крові β1-дефензину та кателіцидину LL-37. Найбільш низькі значення LL-37 були встановлені в дітей, хворих на пневмонію, викликану Streptococcus pneumoniae. Проведений аналіз вмісту метаболітів вітаміну D у сироватці крові показав, що в дітей, хворих на позалікарняну пневмонію, концентрація 25-гідроксивітаміну D була в 1,4 раза нижчою порівняно зі здоровими дітьми (р < 0,05). Установлений дефіцит метаболітів вітаміну D у дітей раннього віку, хворих на позалікарняну пневмонію, є значущим патогенетичним фактором дефіциту кателіцидину LL-37 у сироватці крові, що підтверджувалося зниженням у даній когорті хворих в 3,7 раза процентного вмісту LL-37 щодо метаболітів вітаміну D.
Проведено комплексное обследование 204 детей в возрасте от 2 месяцев до 3 лет с внебольничной пневмонией. Установлено, что у детей раннего возраста, больных внебольничной пневмонией, ведущим этиологическим фактором являются бактерии Streptococcus pneumoniae (36,8 %). Содержание эндогенных антимикробных пептидов определено в сыворотке крови 20 детей раннего возраста, больных внебольничной пневмонией, и 17 детей группы контроля. Доказано, что развитие внебольничной пневмонии у детей раннего возраста происходит на фоне снижения содержания в сыворотке крови β1-дефензина и кателицидина LL-37. Наиболее низкие значения LL-37 были установлены у детей, больных пневмонией, вызванной Streptococcus pneumoniae. Проведенный анализ содержания метаболитов витамина D в сыворотке крови показал, что у детей, больных внебольничной пневмонией, концентрация 25-гидроксивитамина D была в 1,4 раза ниже по сравнению со здоровыми детьми (р < 0,05). Установленный дефицит метаболитов витамина D у детей раннего возраста, больных внебольничной пневмонией, является значимым патогенетическим фактором дефицита кателицидина LL-37 в сыворотке крови, что подтверждалось снижением в данной когорте больных в 3,7 раза процентного содержания LL-37 по отношению к метаболитам витамина D.
The complex examination included 204 children with pneumonia at the age of 2 months to 3 years. It is established that in young children who are ill with out-of-hospital pneumonia, the main etiologic factor is bacteria Streptococcus pneumoniae (36,8%). The content of endogenous antimicrobial peptides identified in the serum of 20 young children, patients with pneumonia and 17 children in the control group. It is proved that the development of pneumonia in young children occurs on the background of the content in serum and β1-defenzinu cathelicidin LL-37. The lowest values of LL-37 were identified in children with pneumonia caused by Streptococcus pneumoniae. The analysis of the content of vitamin D metabolites in the serum, showed that children with pneumonia, concentration 25 Hydroxyvitamin D was 1.4 times lower compared with healthy children (р <0,05). Established deficit metabolites of vitamin D in young children with pneumonia serves important pathogenetic factor deficiency cathelicidin LL-37 in serum, which was confirmed by a decrease in this cohort of patients 3.7 times the percentage of LL-37 in relation to the vitamin D metabolites.
Introduction
Acute respiratory infections of lower respiratory tracts — is a leading reason of child-under 5 years di-sease incidence in the world and makes up 34–40 occurrences among 1000 children a year [1]. Pneumonia is also a leading reason for child of young age deaths in the world. Every year it takes approximately 1,4 million children under 5 years old [2]. An important role in supporting a barrier function of respiratory epithelium and, as a cause, in avoidance of inflammatory diseases of respiratory tracts, play endogenous opiate peptides, in particular: defensin and cathelicidin LL-37, which are secreted by epithelial cell, neutrophils, monocytes and lymphocytes [3, 4]. These peptides are non-specific factors of humoral immunity and display a line of actions, including endotoxin-neutra-lizing and immunomodulatory action, and also provi-ding protection against a wide spectrum of microorganisms: gram-negative and grampositive bacteria, fungus, viruses and the simplest [5]. The relevance of antimicrobial peptides to protect the host’s organism from infection was illustrated on animal models and acknowledged by clinical observations, which demonstrate the change of their expression in cases of diffe-rent diseases of respiratory tract [6]. The activation of NEUTS during infectious and inflammatory processes leads to a quick release of defensins, which later are found in plasma and other organism liquids. Cytokine and defensin activation disorder leads to penetration of originator even in small amounts of it [7].
Cathelicidin has an important role in an innate immunity in protection from bacterial infections, deve-loping antimicrobial activity against gram-negative and gram-positive bacteria, fungus, some viruses and the simplest, and also doing a synergetic antimicrobial effect along with the defensins. The change of concentration LL-37 in blood serum is seen in a range of diseases, including inflammatory diseases of respiratory tracts [8, 9].
Defensin and cathelicidin output is strengthened by vitamin D [10]. Nowadays there is a proof, that 1,25(OH)2D regulates the efficiency of immunity response and has a anti-inflammatory action [11]. This way vitamin D strengthens organism’s protection against bacterial infections [12]. A connection between vitamin D deficiency and the frequency of respiratory disease development was found [13, 14]. In V. Wayse and his coauthors’ study (2004) a raise of severe infection of lower respiratory tracts development risk among children with subclinical vitamin D deficiency was shown [15].
The purpose: to determine intension of endogenic antimicrobial peptides among children of young age, children sick with pneumonia and factors, which affect it.
Materials and methods
We did a complex examination of 204 children of age from two months to 3 years (an average age of patients was 1.6 ± 0.3 years), sick with pneumonia.
A mandatory complex of examination included chest organs roentgenography, a general blood test examination, gene-ral urinalysis, microbial examination of oral swab. Examination of microbial spectrum of tunica mucosa biomaterial was conducted before the antimicrobial therapy was prescribed when the child was directed to the in-patient hospital on a bacteriological analyzer VITEK 2 Сompact (ВioMérieux, France) with the use of AES software: Global CLSI-based + Phenotypic.
β1-defensіns content in blood serum was examined with immune enzymometric analysis with the use of commercial kit Defensin Beta 1 (Elisa, Germany). The exa-mination of cathelicidin level LL-37 was conducted by the immune enzymometric analysis with the use of commercial kit LL-37 (Hyculbiotech, Netherlands). The examination of 25-hydroxyvitamin D was conducted by IFA with the help of commercial kit IDS OSTEIA 25-Hydroxy Vitamin D test. The control group included 17 healthy children, resemblant by age.
Received results were processed by the method of variation statistics with the usage of analysis package program Statisticа for Windows 6.0 with calculating of arithmetical mean (M), standard deviation (σ) and ave-rage mistakes (m). To evaluate difference in measurements in comparable groups we used Student’s t-test. The diffe–rences were considered meaningful, if р < 0.05.
Results
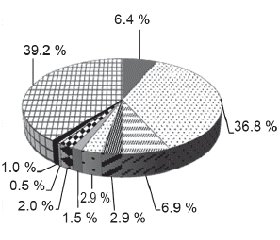
Considering that the main ways of lower respiratory tracts infection of children in young age are oral aspiration and breathing in microbial aerosol, and also, given information from the literature that the respiratory microflora has the same structure, and biomass of which is decreasing from upper to lower tract [16, 17], we did an analysis of oral microbiological “scenery” features of children in young age, who are sick with out of hospital pneumonia. According to the results of microbiological examination we established diagnostically meaningful colonization of upper respiratory tracts by pathogenic microflora with 124 (60.8 %) out of 204 kids (fig. 1). Microflora that was dominating among children who were sick with out-of hospital pneumonia, was Streptococcus pneumoniae — 75 children (36.8 %). Almost six times less we saw gramnegative bacteria Klebsiella pneumoniae — 14 patients (6.9 %) and Haemophillus influenzaе — 13 kids (6.4 %). Other origins were seen in rare cases.
/10-1.gif)
According to this, children of young age, who were sick with out-of hospital pneumonia, had colonization of upper respiratory tracts by Streptococcus pneumoniae bacteria.
It is well-known, that Streptococcus pneumoniae is facultative origin, which inhabits nasopharynx of a person, where it is exposed to a set of antimicrobial peptides, which are a part of innate immunity response. Antimicrobial peptides connect with negatively charged teichoic acids on the membranes of Gram-positive bacteria through electrostatic interaction, which leads to the lysis of microbial cells, creating a first line of defense [18, 19]. That’s why it is possible that the susceptibility of strains of Streptococcus pneumoniae to antimicrobial peptides has a certain role in determining their ability to colonize [20].
So the next step of our work was to determine endo-genous antimicrobial peptides in the blood serum of 20 infants, sick with out-of hospital with pneumonia (tab. 1).
The choice of β1-defensins as a subject of our research is because it is the main factor of innate immunity and so, the antimicrobial barrier system MALT. Defensins take part in all the phases of lungs response, including the initial pathogen destruction. The peptide β1-defensins predominantly shows activity regarding Moraxella catarrhalis and Streptococcus pneumoniae, as well as has a special meaning in avoiding transition of commensal bacteria to opportunistic pathogens [21].
The study of β1-defensins content in blood serum of infants, sick with out-of hospital pneumonia, showed the presence of tendency to reduction of it’s level compared to control group (tab. 1). It is possible, that the received result is conditioned by wide сconstitutively expression β1-defensins with the help of epithelial cells and takes part in innate antimicrobial protection. At the same time, the induction of expression of specified antimicrobial peptide because of inflammatory stimulus is barely happening [22]. Moreover, if other defensins can directly recognize specific lipids in the pathogen membranes [23, 24], then β1-defensin becomes active only when conformation changes after the reduction of its disulfide bonds [25, 26]. In addition, the production of β1-defensin in the epithelial cells of respiratory tracts is reducing in the condition of acidosis [27], which naturally is developed among children, sick with pneumonia [28].
In the course of further work, we investigated the content in the blood serum of children who were under observation, catelicidin LL-37. The multifunctional role of LL-37 is realized due to its ability to recognize and interact with various molecular targets and immune cells [22]. In addition, LL-37 modulates innate immunity by stimulating macrophages to phagocytic bacteria [29]. In addition to antimicrobial activity, LL-37 also activates mechanical features, such as permeability and bacterial uptake by epithelial cells [4].
As a result of the study, it was found that the LL-37 content in the group of infants with out-of hospital pneumonia was 3 times lower than the control group. The lowest values of LL-37 were found in children with pneumococcal pneumonia. It is possible to assume that a decrease in the content of LL-37 in the blood serum of infants is one of the main causes of increased susceptibility to Streptococcus pneumoniae. One possible factor contributing to the decrease in LL-37 activity is that the cations of specific antimicrobial peptide interact with mucin anions, which is a component of airway mucus, resulting in a decrease in the antimicrobial activity of LL-37 [30]. It was found that LL-37 induces the virulence of Streptococcus of group A due to increased production of virulence factors, which is mediated by the component of the regulatory system CsrRS [31]. In year 2014 J.J. Velarde and coauthors identified the smallest fragment of LL-37-RI-10 required for binding to CsRS. This fragment can directly bind to the sensory kinase CsrS, which leads to the activation of expression of virulence factors of the microorganism [32]. It was previously found that such a peptide does not possess antibacterial activity [33, 34]. In the opinion of I. Gryllos and coauthors (2008), LL-37 has a paradoxical effect, stimulating the regulated CsrRS expression of the virulence gene, thereby increasing the pathogenicity of group A Streptococcus during an infectious disease. The ability of Streptococcus of group A to perceive and respond to LL-37 may partially explain the susceptibility of humans as a biological species not only to Streptococcus of group A, but also to streptococcal infection in general [31]. Based on the data obtained, it was suggested that in the conditions of LL-37 deficiency an inversion of its action is observed, that is, instead of the expected bactericidal effect, the virulence of the microorganism increases [35].
An additional factor that may influence the expression and activity of LL-37 may be metabolic or respiratory-metabolic acidosis. Abou Alaiwa and coauthors (2014) showed that the antibacterial activity of LL-37 depends on the pH of the airways. At the same time, a decrease in pH from 8 to 6,8 in the airways reduces the activity of LL-37 [36]. It is known that pH modulates the state of human oligomerization of LL-37. At acid pH, LL-37 is monomeric, at physiological pH cathecidin aggregates [37]. In the work of Singh, D. and coauthors (2014), it was found that the enhancement of LL-37 signal transduction by the Toll-like receptor 3 (TLR3) is regulated by pH [38]. Upon acidification by endosomes, the oligomerized LL-37 dissociates into LL-29 (a natural LL-37 fragment lacking the C-terminal part) [39], which is unable to transmit TLR3 signals [38]. In this case, inhibition of cathepsins, which includes proteases, whose activity is activated by endosome acidification, resulted in an increase in the half-life of LL-37 from cells [38].
Discussion
It is known that vitamin D plays an important role in regulating LL-37 expression. Respiratory epithelial cells convert vitamin D into its active metabolite calcitriol, which has a 100 times greater affinity for the vitamin D receptor than calcidiol [40]. The process of formation of calcitriol is catalyzed by the enzyme α1-hydroxylase, that is present in the mitochondria of renal tubular cells [41]. The interaction of calcitriol with epithelial cells of the respiratory tract leads to active synthesis of catelicidin protein, which prevents penetration of pathogens into the lower respiratory tract [40].
In the works of P.T. Liu and coauthors (2006) describes the vitamin-D-dependent pathway of the TLR2/1-associated pathway for the synthesis of antimicrobial peptides. It has been shown that activation of human macrophage TLR with increased expression of the vitamin D receptor, promotes the induction of LL-37 expression. According to the authors, the findings confirm the relationship between TLR and vitamin D-mediated congenital immunity and suggest that differences in a person's ability to produce vitamin D affect susceptibility to microbial infection [42]. It was shown by the researchers from New Zealand (2011), that in patients with pneumonia, severe 25-hydroxyvitamin D-deficiency (< 30 nmol/L) correlated closely with a higher 30-day mortality compared to patients with a sufficient level (> 50 nmol/L) [43].
Considering that the low level of vitamin D supply is associated with a high risk of developing respiratory tract infections [44, 45], the next step of our work was to determine the content of vitamin D metabolites in the serum of children in the observation groups (tab. 1).
The analysis of the content of vitamin D metabolites in blood serum showed that among children, sick with out-of hospital pneumonia, the concentration of 25-hydroxyvitamin D was 1.4 times lower than in healthy children and averaged 75.0 ± 10.0 mIU/ml vs 104.8 ± 6.7 mIU/ml, accordingly (p < 0.05). Taking into account that vitamin D induces LL-37 expression; we determined the ratio of LL-37 to 25-hydroxyvitamin D. It was found that in the group of children with pneumonia, a 3.7-fold decrease in the percentage of LL-37 in relation to vitamin D (0.12 ± ± 0.04 % against 0.44 ± 0.12 %, accordingly, p < 0.05). So, on the background of low levels of vitamin D among children with pneumonia, there was not enough LL-37 synthesis.
One of the pathogenetic mechanisms for reducing calcitriol levels is a decrease in the activity of 1α-hydroxylase [46]. In numerous experimental studies, it was shown that the development of metabolic acidosis suppresses the synthesis of 25-hydroxyvitamin D3-1α-hydroxylase in the proximal tubules of the kidneys by inhibiting parathyroid hormone-dependent adenylate cyclase, which leads to a decrease in serum vitamin D levels [47–49] and , as a consequence, a decrease in the synthesis of LL-37 [50].
Conclusions
1. The development of out-of hospital pneumonia among infants occurs at the background of a low blood serum level of a number of endogenous antimicrobial peptides (β1-defensin and LL-37).
2. A significant pathogenetic factor of the deficiency of cathelicidin LL-37 in the blood serum of infants with out-of hospital pneumonia is the deficiency of vitamin D metabolites.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. McIntosh K. Community-acquired pneumonia in children. New England Journal of Medicine. 2002;346(6):429-437. doi: 10.1056/NEJMra011994.
2. World Health Organization (WHO). Pneumoni a factsheet. Factsheet № 331. Reviewed November 2013. Available at: www.who.int/mediacentre/factsheets/fs331/en/.
3. Wu Rui-Qing, Dun-Fang Zhang, Eric Tu.The mucosa limmune systemin the oral cavity- an orche straof T cell diversity. Int J Oral Sci. 2014;6(3):125-32. doi:10.1038/ijos.2014.48.
4. Tyrnova EV, Aleshina GM, Yanov YuK, Kokryakov VN. Expression of the β-defensin-1 and -2 and catelicidin LL-37 in the respiratory mucosa. Evaluation of the expression of beta-defensin-1 and -2 human genes and catelicidin LL-37 in the mucosa of the upper respiratory tract]. Tsitokiny i vospaleniye. 2014;13(2):89-95.
5. Aleshina GM, Kokryakov VN, Shamova OV. Modern concept of antimicrobial peptides as molecular factors of immunity. Meditsinskiy akademicheskiy zhurnal. 2010;4:149-160.
6. Hiemstra PS, Amatngalim GD, Van Der Does AM. Antimicrobial Peptides and Innate Lung Defenses: Role in Infectious and Noninfectious Lung Diseases and Therapeutic Applications. CHEST Journal. 2016;149(2):545-551. doi:10.1378/chest.15-1353.
7. Miroshnichenko YuA, Shestopalov AV, Smolyaninova LP. The role of factors of congenital immunity of the mucous membrane of the reproductive tract. Zhurnal fundamentalnoy meditsinyi i biologii. 2013;1:11.
8. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(4):389-395. doi:10.1038/415389a.
9. Zaiou М, Nizet V, Gallo RL. Antimicrobial and Protease Inhibitory Functions of the Human Cathelicidin (hCAP18/LL-37) Prosequence. The Journal of Investigative Dermatology. 2003;120(5):810-816. doi:1523-1747.2003.12132.x.
10. Hansdottir S, Monick MM, Hinde SL. Respiratory epithelial cells convertinactive vitamin D toits active form: potential effects on hostdefense. J Immunol. 2008;181(10):7090-9. doi: 10.4049/jimmunol.181.10.7090.
11. Zaharova IN, Yablochkova SV, Dmitrieva YuA. Known and unknown effects of vitamin D. Voprosyi sovremennoy pediatrii. 2013;2:20-25.
12. Gorgoni B, Maritano D, Marthyn PC. EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulationof IL-12 p40 and p35 mRNAs in macrophages. Journal of Immunology. 2002;168(8):4055-62. doi: 10.4049/jimmunol.168.8.4055.
13. Yim S, Dhawan P, Ragunath C. Induction of cathelicidin in normaland CF bronchial epithelial cells by 1,25 dihydroxy Vitamin D3. Journal of Cystic Fibrosis. 2007;6(6):403-410. doi: 10.1016/j.jcf.2007.03.003.
14. Belderbos ME, Houben ML, Wilbrink B. Cord bloodvitamin D deficiencyis associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127(6):1513-20. doi: 10.1542/peds.2010-3054.
15. Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563-7. doi: 10.1038/sj.ejcn.1601845.
16. Pikuza OI, Samorodnova EA. Modern features of community-acquired pneumonia in young children. Prakticheskaya meditsina. 2013;6(75):35-41.
17. Charlson ES, Bittinger K, Haas AR. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Amer J Respir And Crit Care Med. 2011;184(8):957-63. doi:10.1164/rccm.201104-0655OC.
18. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238-50. doi:10.1038/nrmicro1098.
19. Burton MF, Steel PG. The chemistry and biology of LL-37. Nat Prod Rep. 2009;26:1572-84. doi: 10.1039/b912533g.
20. Habets MG, Rozen DE, Brockhurst MA. Variation in Streptococcus pneumoniae susceptibility to human antimicrobial peptides may mediate intraspecific competition. Proc Biol Sci. 2012 Sep 22;279(1743):3803-11. doi: 10.1098/rspb.2012.1118.
21. Abaturov AE, Gerasimenko ON, Vyisochina IL, Zavgorodnyaya NYu. Defenzinyi i defenzin-zavisimyie zabolevaniya [Defensins and defensin-dependent diseases]. O.: VMV; 2011. 264 p.
22. Doss M, White MR, Tecle T, Hartshorn KL. Human defensins and LL-37 in mucosal immunity. Journal of leukocyte biology. 2010;87(1):79-92. doi: 10.1189/jlb.0609382.
23. De Medeiros LN, Angeli R, Sarzedas CG. Backbone dynamics of the antifungal Psd1 pea defensin and its correlation with membrane interaction by NMR spectroscopy. Biochim Biophys Acta. 2010;1798:105-113. doi: 10.1016/j.bbamem.2009.07.013.
24. Shenkarev ZO, Gizatullina AK, Finkina EI. Heterologous expression and solution structure of defensin from lentil Lens culinaris. Biochem Biophys Res Commun. 2014;451:252-257. doi: 10.1016/j.bbrc.2014.07.104.
25. Schroeder BO, Wu Z, Nuding S. Reduction of disulphide bonds un masks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419-23. doi: 10.1038/nature09674.
26. Raschig J, Mailänder-Sanchez D, Berscheid A. Ubiquitously expressed Human Beta Defensin 1 (hBD1) forms bacteria-entrapping netsin a redox dependent mode of action. Plo Spathogens. 2017;13(3):S1006261. doi: 10.1371/journal.ppat.1006261.
27. Nakayama K, Jia YX, Hirai H. Acid stimulation reduces bactericidal activity of surface liquid in cultured human air way epithelial cells. American journal of respiratory cell and molecular biology. 2002;26(1):105-113. doi: 10.1165/ajrcmb.26.1.4425.
28. Shabalov NP. Pneumonia in young children. Lechaschiy vrach. 2003;2:16-22.
29. Wan M, vander Does AM, Tang X. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J Leukoc Biol. 2014;95:971-81. doi: 10.1189/jlb.0513304.
30. Felgentreff K, Beisswenger C, Griese M. The antimicrobial peptide cathelicidin interacts with air way mucus. Peptides. 2006;27(12):3100-06. doi: 10.1016/j.peptides.2006.07.018.
31. Gryllos I, Tran-Winkler HJ, Cheng MF. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc Natl Acad Sci USA. 2008;105(43):16755-60. doi: 10.1073/pnas.0803815105.
32. Velarde JJ, Ashbaugh M, Wessels MR. The human antimicrobial peptide LL-37 binds directly to CsrS, a sensor his tidineki nase of group A Streptococcus, to activ at eexpression of virulence factors. J Biol Chem. 2014;289:36315-24. doi: 10.1074/jbc.M114.605394.
33. Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem. 2008;283:32637-43. doi: 10.1074/jbc.M805533200.
34. Epand RF, Wang G, Berno B, Epand RM. Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob Agents Chemother. 2009;53:3705-14. doi: 10.1128/AAC.00321-09.
35. Wang G, Epand RF, Mishra B. Decoding the functional roles of cationic side chains of the major antimicrobial region of human cathelicidin LL-37. Antimicrob Agents Chemother. 2012;56:845-56. doi: 10.1128/AAC.05637-11.
36. Abou Alaiwa MH, Reznikov LR, Gansemer ND. PH Modulates the activity and synergis mofthe airway surface liquid antimicrobials beta-defensin-3 and LL-37. Proc Natl Acad Sci USA. 2014;111:18703-8. doi: 10.1073/pnas.1422091112.
37. Johansson J, Gudmundsson GH, Rottenberg ME. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718-24. doi: 10.1074/jbc.273.6.3718.
38. Singh D, Vaughan R, Kao CC. LL-37 peptide enhancement of signal transductionby toll-like receptor 3 is regulated by pH: identification of a peptide antagonist of LL-37. J Biol Chem. 2014;289:27614-24. doi: 10.1074/jbc.M114.582973.
39. Yamasaki K, Schauber J, Coda A. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicid in sinskin. FASEB Journal. 2006;20:2068-80. doi: 10.1096/fj.06-6075com.
40. Zaharova IN. Vitamin D: unknown about the known. Rossiyskiy meditsinskiy zhurnal. 2015;3:118.
41. Zaharova IN, Dmitrieva YuA, Yablochkova SV. Modern view on the metabolism and physiological effects of vitamin D in the human body. Vestnik AGIUV 2013;2:27-31.
42. Liu PT, Stenger S, Li H. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770-3. doi: 10.1126/science.1123933.
43. Leow L, Simpson T, Cursons R. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology. 2011;16(4):611-6. doi: 10.1111/j.1440-1843.2011.01924.x.
44. Laaksi I, Ruohola JP, Tuohimaa P. An association of serum vitamin D concentrations < 40 nmol/l with acute respiratory tract infection in young Finnishmen. Am J Clin Nutr. 2007;86(3):714-7. doi: 10.3390/nu5072502.
45. Karatekin G, Kaya A, Salihoglu O. Association of subclinica lvitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63(4):473-7. doi: 10.1038/sj.ejcn.1602960.
46. De Boer IH. Vitamin D and glucose metabolism in chronic kidney disease. Current opinion in nephrology and hypertension. 2008;17(6):566. doi: 10.1097/MNH.0b013e32830fe377.
47. Lee SW, Russell J, Avioli LV. 25-hydroxycholecalciferol to 1, 25-dihydroxycholecalciferol: conversion impaired by systemic metabolic acidosis. 1977;195(4282):994-6. doi: 10.1126/science.841324.
48. Kawashima H, Kraut JA, Kurokawa K. Metabolic acidosis suppresses 25-hydroxyvitamin in D3-1alpha-hydroxylase in the rat kidney. Distinct site and mechanism of action. J Clin Invest. 1982;70:135-40. doi: 10.1172/JCI110586.
49. Chan YL, Sardie E, Mason RS, Posen S. The effect of metabolic acidosis on vitamin D metabolism and bone histology in uremic rats. Calcif Tissue Int. 1985;37:158-64. doi: 10.1007/BF02554835.
50. Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacerium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060-3. doi: 10.4049/jimmunol.179.4.2060.

/10-1.gif)
/10-2.gif)