Тучные клетки
Тучные клетки представляют собой резидентные фагоциты, способные осуществлять внутриклеточный киллинг активированными кислородсодержащими метаболитами (АКМ), внеклеточный киллинг, используя формирование внеклеточных ловушек и высвобождение антимикробных пептидов. Кроме того, тучные клетки обладают уникальной способностью быстро высвобождать такие вазоактивные и иммуностимулирующие медиаторы, как фактор некроза опухоли β (TNF-β), гистамин, триптаза и химаза, во внеклеточную среду, в том числе и во время пневмонии, вызванной Staphylococcus aureus [3, 33].
Нейтрофилы
Нейтрофилы рекрутируются при помощи цитокинов и хемокинов в очаг поражения легких, а нейтрофильное представительство в легочном инфильтрате является отличительной чертой раннего воспалительного ответа на инфицирование золотистым стафилококком [31].
A.N. Spaan и соавт. [39] считают, что нейтрофилы как эффекторные клетки, которые хорошо оснащены для выполнения вне- и внутриклеточного киллинга, играют ключевую роль в неспецифической защите макроорганизма от золотистого стафилококка (табл. 1).
При изучении стафилококковой инфекции Y. Tsuda и соавт. [45] выделили три субпопуляции нейтрофилов: 1) нейтрофилы в покое с фенотипом CD49d–CD11b–, не продуцирующие существенного количества цитокинов и хемокинов; 2) нейтрофилы 1-го типа (N1) с фенотипом CD49d+CD1b–, продуцирующие IL-12 и CCL3; 3) нейтрофилы 2-го типа (N2) с фенотипом CD49d–CD11b+, продуцирующие IL-10 и CCL2.
Роль различных нейтрофильных субпопуляций в развитии стафилококковой инфекции представлена в табл. 3.
Также различают нейтрофилы, циркулирующие в периферическом русле крови, и нейтрофилы, находящиеся в селезенке. I. Puga [29] показала, что нейтрофилы в физиологических условиях локализуются в перимаргинальной зоне селезенки, а во время системного инфекционного процесса они преимущественно концентрируются в маргинальной зоне и могут появляться в фолликулярной зоне селезенки. Фенотип нейтрофилов селезенки или нейтрофилов хелперов В-клеток (B cell-helper neutrophils — NBH cells) отличается от фенотипа циркулирующих конвенциональных нейтрофилов (conventional neutrophils — NC). Клетки NBH, в свою очередь, образовывают две субпопуляции нейтрофилов — NBH1 и NBH2 (табл. 4). Нейтрофилы NC высоко экспрессируют молекулы CD15 и CD16; NBH1-клетки отличаются промежуточным уровнем экспрессии CD15 и CD16, а NBH2-клетки характеризуются низким уровнем экспрессии CD15 и CD16 [50].
/168-1.jpg )
/169-1.jpg )
/170-1.jpg )
/171-1.jpg )
Исследования функциональных особенностей выделенных субпопуляций нейтрофилов позволят обеспечить новое понимание вклада нейтрофилов в патогенез инфекционно-воспалительных заболеваний и станут основой для разработки новых способов лечения, направленных на дифференцированную модуляцию активности различных субпопуляций нейтрофилов для достижения эффективного клиренса патогенных агентов [36].
O. Kamenyeva и соавт. [16] подчеркивает, что в естественных условиях стафилококковой инфекции рекрутинг нейтрофилов в очаг поражения происходит в виде двух волн. Первая волна обусловлена притоком нейтрофилов из периферической крови, как правило, за счет действия эпителиальных цитокинов (IL-1β и TNF), а вторая осуществляется за счет мобилизации нейтрофилов из костного мозга. Зрелые нейтрофилы экспрессируют Ly6Ghi, CXCR2, CXCR4 и локализуются в красной пульпе селезенки и костном мозге. Удержание в континууме костного мозга и высвобождение из него регулируются экспрессируемыми на нейтрофилах рецепторами хемокинов CXCR2 и CXCR4. Активация CXCR4 лигандом CXCL12, в основном экспрессированным остеобластами, удерживает нейтрофилы в костном мозге, а возбуждение CXCR2 лигандами CXCL1 и CXCL2, которые преимущественно экспрессируются эндотелиальными клетками, обусловливает высвобождение нейтрофилов из костного мозга. При физиологических условиях активность CXCR4/CXCL12 преобладает над активностью CXCR2-ассоциированных сигнальных путей и обусловливает то, что большую часть своей жизни нейтрофилы проводят в костном мозге, и только около 2 % нейтрофилов находятся в периферическом русле крови [30, 31, 35, 40]. Во время воспаления нейтрофилы из костного мозга мобилизуются в кровь и мигрируют в сторону источника продукции СХС-хемокинов и других медиаторов воспаления, высвобождаемых пораженными клетками или активными иммуноцитами. Многие медиаторы, высвобождаемые самими нейтрофилами, являются нейтрофильными хемоаттрактантами, поэтому нейтрофилы могут рекрутировать другие нейтрофилы (табл. 5) [35].
/172-1.jpg )
Компоненты стафилококковой стенки, в первую очередь пептидогликаны (peptidoglycan — PGN), активируют продукцию компонента комплемента С5а, который обладает мощной способностью привлекать нейтрофилы. Бактериальные токсины (например, N-формил-пептиды или фенолсолютабные модулины) золотистого стафилококка способны непосредственно вербовать нейтрофилы [28].
Одновременно нейтрофилы попадают в региональные лимфатические узлы [17], где они поддерживают дифференцировку наивных Т-лимфоцитов в Th1- и Th17-клетки [22]. В лимфоузлах активированные нейтрофилы непосредственно ингибируют дифференцировку наивных B-клеток в секретирующие антитела клетки за счет продукции TGF-β1 [16].
Для обеспечения миграции нейтрофилов из кровеносного русла необходима активация молекул адгезии на эндотелиоцитах. Данная нейтрофильная миграция в основном происходит в посткапиллярных венулах [35].
Нейтрофилы играют ключевую роль в процессе саногенеза стафилококковой пневмонии. Так, при фармакологическом истощении нейтрофильной популяции уровень летальности при стафилококковой пневмонии у мышей достигает 90 %. У мышей с нейтрофильным истощением снижен уровень активности бактериального клиренса, но, как ни странно, это не сопровождается увеличением риска возникновения бактериемии [32].
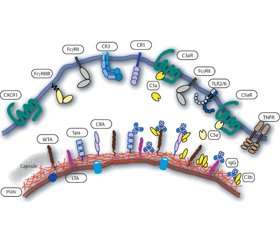
Для эффективного взаимодействия нейтрофилов с инфекционными патогенами и возбуждения фагоцитоза необходимо предварительное связывание бактерий с опсонинами, которые распознаются специфическими рецепторами нейтрофилов. Основными содержащимися в крови опсонинами являются иммуноглобулины и компоненты системы комплемента, которые легко связываются как с бактериями Staphylococcus aureus, так и со специфическими иммуноглобулиновыми рецепторами FcR и рецепторами комплемента CRS, расположенными на внешней поверхности цитоплазматической мембраны нейтрофилов (рис. 1) [46].
Нейтрофилы для бактериального киллинга используют механизмы фагоцитоза, нейтрофильные внешние ловушки, АКМ, активированные азотсодержащие метаболиты (ААМ), антимикробные пептиды [20]. Необходимо отметить, что фагоцитоз нейтрофилов и макрофагов имеет функциональные отличия (табл. 6) [24].
/173-1.jpg )
Нейтрофилы характеризуются более быстрым темпом фагоцитоза, более высокой интенсивностью генерации АКМ. Нейтрофилы при интернализации для восстановления цитоплазматической мембраны не используют внутренние мембранные резервы, в то время как макрофаги восполняют интернализированную во время фагоцитоза часть цитоплазматической мембраны внутриклеточной мембраной эндоплазматического ретикулума. Считается, что во время данной замены цитоплазматической мембраны мембраной эндоплазматического ретикулума происходит высвобождение цитокинов [24]. Нейтрофилы способны одновременно поглотить более 50 бактерий. Нейтрофилы являются чрезвычайно эффективными фагоцитами и могут интернализовать IgG-опсонизированные латексные шарики менее чем через 20 секунд после их взаимодействия [46].
Рекрутированные в очаг поражения легких нейтрофилы фагоцитируют инвазивные бактерии и изолируют их в фагосоме, в которой содержатся высокие концентрации антимикробных пептидов, протеолитических ферментов и АКМ, генерируемых НАДФН-оксидазой [6]. Такие АКМ, как H2O2 и HOCl, антимикробные пептиды и протеолитические ферменты осуществляют внутриклеточный киллинг бактерий Staphylococcus aureus. Внеклеточный киллинг бактерий нейтрофилы производят при помощи ААМ и нейтрофильных внеклеточных ловушек (НВЛ) (рис. 2) [20].
В ответ на инфицирование бактериями Staphylococcus aureus нейтрофилы очень быстро, в течение 5–60 минут, формируют НВЛ. –Установлено, что для формирования НВЛ необходимы живые бактерии. Основным фактором вирулентности, индуцирующим формирование НВЛ, является лейкоцидин Panton Valentine. IgA-опсонизированные бактерии Staphylococcus aureus также быстро инициируют образование НВЛ, вероятно, из-за высокого уровня генерации АКМ [1, 7, 28].
Нейтрофилы в отличие от макрофагов способны к фагоцитированию бактерий Staphylococcus aureus, ассоциированных с биопленкой [43]. –Развивающиеся биопленки более чувствительны к нейтрофильной атаке, чем зрелые биопленки. Таким образом, чем раньше происходит нейтрофильная атака, тем лучше ее антибактериальный результат [12]. Известно, что формирование биопленки способствует персистенции бактерий Staphylococcus aureus [47].
Нейтрофилы после ликвидации фагоцитированных бактерий погибают путем апоптоза. Поглощение апоптотических клеток макрофагами предопределяет разрешение процесса воспаления и развитие реконвалесценции заболевания [4]. Таким образом, эффероцитоз макрофагами апоптотических нейтрофилов, предотвращая лизис нейтрофилов, подавляет иммунные реакции и обусловливает активацию противовоспалительных сигнальных путей [10].
Бактерицидное действие нейтрофилов обусловливает гибель подавляющего большинства вторгшихся бактерий Staphylococcus aureus, однако некоторые бактерии могут уклоняться от механизмов нейтрофильного киллинга. Действительно, продемонстрировано, что бактерии Staphylococcus aureus подавляют активность нейтрофилов: нарушают хемотаксис, ингибируют активность фагоцитоза, продукцию антимикробных пептидов и др. [42].
Однако бактерии Staphylococcus aureus, особенно MRSA, после фагоцитоза могут индуцировать лизис нейтрофилов [9, 44], что является серьезной саногенетической проблемой. Выраженный MRSA-индуцированный лизис нейтрофилов может привести к неблагоприятному течению и летальному исходу заболевания, вызванного Staphylococcus aureus.
Бактерии Staphylococcus aureus обладают широким спектром механизмов уклонения от антибактериального действия нейтрофилов. А сложность сети взаимодействий между бактериями золотистого стафилококка и человеческим организмом объективно препятствует получению достоверных результатов при проведении простых экспериментальных исследований инфекционного процесса и затрудняет создание новых терапевтических средств, модулирующих активность нейтрофилов [42].
Таким образом, нейтрофилы являются центральным клеточным компонентом неспецифической системы защиты макроорганизма от бактерий Staphylococcus aureus, эффективность функционирования которого определяет течение и исход стафилококковой пневмонии.
Дендритные клетки
Дендритные клетки были идентифицированы как отдельная клеточная субпопуляция, основной функцией которой является презентация антигена, Ральфом Стейнманом в 1973 году. В настоящее время выделено четыре основных субпопуляции DC, которые отличаются друг от друга как фенотипом, так и функциональными возможностями. Различают две субпопуляции конвенциональных или миелоидных DC (conventional DC — сDC), а также плазмацитоидные DC (plasmacytoid DC — pDC) и DC моноцитарного происхождения (monocyte-derived DC — moDC) [11, 27].
Характеристика DC легочной ткани представлена в табл. 7.
Конвенциональные дендритные клетки
Конвенциональные DC (сDC, или миелоидные DC) участвуют в регуляции реакции Т-клеток на инфекционный агент, в частности после миграции в регионарный лимфатический узел, презентируют антиген Т-лимфоцитам и предопределяют канализированность цитодифференцировки наивных Т-клеток. D. Schindler и соавт. [37] установили, что сDC1 мобилизуются и активно привлекаются в инфицированную ткань во время инфекционного процесса, вызванного бактериями Staphylococcus aureus. Авторами было показано, что сDC1 играют ключевую роль в процессе специфического клиренса бактерий золотистого стафилококка, и истощение данной клеточной субпопуляции у экспериментальных животных увеличивает вероятность развития бактериемии и танатогенеза у мышей, инфицированных Staphylococcus aureus. Также трансфер сDC1 инфицированным Staphylococcus aureus мышам способствует улучшению бактериального клиренса. Однако сDC1 не играют существенной роли в непосредственном бактериальном киллинге, а бактерии Staphylococcus aureus выживают и даже могут размножаться в сDC1. Представляет интерес тот факт, что нейтрофилы, выделенные из легких мышей с пониженным содержанием DC, содержат значительно более высокие количества внутриклеточных жизнеспособных бактерий Staphylococcus aureus, чем нейтрофилы, изолированные от мышей с нормальным содержанием DC. Авторы считают, что способность рекрутированных нейтрофилов выполнять внутриклеточный киллинг бактерий Staphylococcus aureus зависит от наличия DC. С учетом того, что DC являются основным источником IL-12, дефицит DC может привести к недостаточности IL-12, который играет ключевую роль в саногенезе стафилококковой пневмонии [19, 23].
Jun-O Jin [15] продемонстрировал, что BDCA1+cDC представляют собой уникальную субпопуляцию, которая может индуцировать иммунные ответы против бактерий Staphylococcus aureus. Клетки BDCA1+cDC могут поглощать бактерии Staphylococcus aureus и усиливать как экспрессию костимулирующих молекул, так и продукцию провоспалительных цитокинов. Кроме того, клетки BDCA1+cDC в ответ на инфицирование Staphylococcus aureus экспрессируют высокие уровни молекул главного класса гистосовместимости (major histocompatibility complex — MHC) I и II класса, способствуют пролиферации CD4+Th1-, CD8+Тс1-T-клеток и продукции IFN-γ. Интересен тот факт, что способность данных cDC активировать Th1-реакцию связана с высоким уровнем экспрессии TLR2 и скавенджер-рецептора A (scavenger receptor A — SR-A), в то время как у BDCA3+CD16+cDC экспрессия SR-A крайне низкая.
pDC
Плазмацитоидные DC (pDC) представляют собой основные продуценты IFN-α в организме человека [41]. pDC являются субпопуляцией лейкоцитов, несущих на поверхности своей цитоплазматической мембраны Fcγ- и Fcε-рецепторы, возбуждение которых оказывает разнонаправленное действие на чувствительность эндосомальных TLR к внутриклеточно локализованным микробным нуклеиновым кислотам. Патогенные бактерии индуцируют pDC, что сопровождается секрецией IFN-α, TNF-α и IL-6 [21]. Staphylococcus aureus-индуцированная секреция IFN-α pDC обусловлена активацией TLR7 и TLR9 нуклеиновыми кислотами патогена. Вызывает интерес то, что другие внеклеточные бактерии (коагулазоотрицательные стафилококки) в отличие от золотистого стафилококка не приводят к продукции IFN-α плазмацитоидными DC. Активация pDC происходит антигенспецифическим способом, то есть для индукции синтеза IFN-α необходимы спе–цифические антистафилококковые антитела, принадлежащие к IgG. Так, быстрое проникновение бактерий Staphylococcus aureus в pDC опосредуется IgG. Отсутствие IgG или нейтрализация рецептора FcγRIIA на pDC блокирует бактериальный эндоцитоз и, таким образом, предотвращает доступ бактериальных нуклеиновых кислот к эндосомным TLR, что предупреждает продукцию IFN-α. Считают, что активацию pDC опосредуют специфические антитела, принадлежащие к подклассам IgG: IgG1 и IgG3. Из-за постоянного взаимодействия организма человека и бактерий Staphylococcus aureus в сыворотке крови человека обычно содержатся антистафилококковые антитела, принадлежащие к IgG, что объясняет предрасположенность pDC реагировать образованием IFN-α. В обычных условиях pDC участвуют во вторичной реакции организма на патоген, то есть активация pDC инициируется поглощением стафилококковых иммунных комплексов, связанных с IgG или IgE. Однако поверхностный протеин A (surface protein A — SpA) бактерий Staphylococcus aureus может активировать pDC и при отсутствии специфических антител. Staphylococcus aureus-индуцированная активация pDC усиливает экспансию поликлональных B-клеток и способствует пролиферации супрессивных IL-10-продуцирующих B-клеток. Известно, что истощение В-клеток сопровождается увеличением продукции IFNγ в ответ на инфицирование бактериями Staphylococcus aureus. Staphylococcus aureus-индуцированная активация pDC может способствовать пролиферации IL-10-продуцирующих Treg-клеток.Таким образом, активация pDC является элементом вторичного иммунного ответа на бактерии Staphylococcus aureus. В то же время бактерии Staphylococcus aureus используют pDC для индукции антигеннезависимой дифференцировки IL-10-продуцирующих плазмокластов, уклоняясь от механизмов элиминации макроорганизма [2, 25].
Установлено, что индукция синтеза IFN-α pDC лигандом TLR9 CpG ДНК способствует выздоровлению от пневмонии, вызванной бактериями Staphylococcus aureus [34]. Представляет интерес тот факт, что агонисты TLR7 и TLR9, вызывающие продукцию IFN-α, подавляют продукцию IL-17 [5]. Ингибирующее действие IFN-α на продукцию –IL-17, вероятно, имеет опосредованный характер и обусловлено тем, что IFN-α индуцирует синтез –IL-17-ингибирующего цитокина IL-27 [3].
moDC
V. Frodermann и соавт. [8] представили доказательства участия moDC в инфекционном процессе, вызванном бактериями Staphylococcus aureus. Показано, что PAMP (липопротеины, тейхоевые кислоты и PGN) бактерий Staphylococcus aureus активируют CD14- и CD36-независимым способом TLR2 моноцитов и макрофагов и moDC. Активация TLR2 моноцитов и макрофагов возбуждает PI3K-ассоциированный сигнальный путь, который приводит к продукции IL-10, в то время как активация TLR2 moDC сопровождается преимущественно продукцией IL-12 и IL-23, что индуцирует устойчивый Th1-/Th17-ответ [8, 14, 18].
Продукты бактерий Staphylococcus aureus модулируют функционирование DC за счет киллинга DC, ингибирование Th1-ответа, индуцирование Th2- и Treg-ответов, а также усиление пролиферации B-клеток, продуцирующих IL-10. Так, стафилококковый цитотоксин лейкоцидин A/B (LukAB) опосредует киллинг moDC. Энтеротоксин B (Staphylococcus aureus enterotoxin B — SEB) способствует пролиферации DC, продуцирующих IL-2 и экспрессирующих протеин-4, содержащий домен Т-клеточного иммуноглобулина и муцина (T cell immunoglobulin mucin domain 4 — TIM4), что индуцирует Th2-реакцию. Фенолсолютабные модулины (phenol soluble modulins) ингибируют секрецию провоспалительных цитокинов (TNF-α, IL-12 и -6) и стимулируют секрецию IL-10 IL-10-продуцирующими-DC, что подавляет активность Th1-ответа [49].
Роль дендритных клеток в развитии стафилококковой пневмонии схематично представлена на рис. 3.
Конфликт интересов. Авторы заявляют об отсутствии какого-либо конфликта интересов при подготовке данной статьи.
Список литературы
1. Aleyd E. IgA enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fcα receptor I / E. Aleyd, M.W. van Hout, S.H. Ganzevles et al. // J. Immunol. — 2014, Mar 1. — 192(5). — 2374-83. — doi: 10.4049/jimmunol.1300261.
2. Bekeredjian-Ding I. Plasmacytoid Dendritic Cells: Neglected Regulators of the Immune R esponse to Staphylococcus aureus / I. Beke–redjian-Ding, J. Greil, S. Ammann, M. Parcina // Front. Immunol. — 2014, May 23. — 5. — 238. — doi: 10.3389/fimmu.2014.00238.
3. Bekeredjian-Ding I., Stein C., Uebele J. The Innate Immune Response Against Staphylococcus aureus // Curr. Top. Microbiol. Immunol. — 2015, Dec 15. — doi: 10.1007/82_2015_5004.
4. Bratton D.L., Henson P.M. Neutrophil clearance: when the party is over, clean-up begins // Trends Immunol. — 2011 Aug. — 32(8). — 350-7. — doi: 10.1016/j.it.2011.04.009.
5. Cui F., Meng J., Luo P, Chen P. IFN- alpha blocks IL-17 production by peripheral blood mononuclear cells in patients with chronic active hepatitis B Infection // BMC Infect. Dis. — 2014, Feb 1. — 14. — 55. — doi: 10.1186/1471-2334-14-55.
6. DeLeo F.R., Nauseef W.M. Granulocytic phagocytes // Bennet J.E., Dolin R., Blaser M.J., editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases.
8. This textbook chapter is a comprehensive review of the cell and molecular biology of human neutrophils. — Philadelphia, PA: Elsevier/Saunders, 2014.
7. Delgado-Rizo V. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview / V. Delgado-Rizo, M.A. Martínez-Guzmán, L. Iñiguez-Gutierrez et al. // Front. Immunol. — 2017, Feb 6. — 8. — 81. — doi: 10.3389/fimmu.2017.00081.
8. Frodermann V. A modulatory interleukin-10 response to staphylo–coccal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus / V. Frodermann, T.A. Chau, S. Sayedyahossein et al. // J. Infect. Dis. — 2011, Jul 15. — 204(2). — 253-62. — doi: 10.1093/infdis/jir276.
9. Greenlee-Wacker M., DeLeo F.R., Nauseef WM. How methicillin-resistant Staphylococcus aureus evade neutrophil killing // Curr. Opin. Hematol. — 2015 Jan. — 22(1). — 30-5. — doi: 10.1097/MOH.0000000000000096.
10. Greenlee-Wacker M.C. Clearance of apoptotic neutrophils and resolution of inflammation // Immunol. Rev. — 2016 Sep. — 273(1). — 357-70. — doi: 10.1111/imr.12453.
11. Guilliams M. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny / M. Guilliams, F. Ginhoux, C. Jakubzick et al. // Nat. Rev. Immunol. — 2014 Aug. — 14(8). — 571-8. — doi: 10.1038/nri3712.
12. Günther F. Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN) / F. Günther, G.H. Wabnitz, P. Stroh et al. // Mol. Immunol. — 2009 May. — 46(8–9). — 1805-13. — doi: 10.1016/j.molimm.2009.01.020.
13. Honda T. Neutrophil left shift and white blood cell count as markers of bacterial infection / T. Honda, T. Uehara, G. Matsumoto, S. Arai, M. Sugano // Clin. Chim. Acta. — 2016, Jun 1. — 457. — 46-53. — doi: 10.1016/j.cca.2016.03.017.
14. Hong S.J. Wall teichoic acid is an essential component of Staphylococcus aureus for the induction of human dendritic cell maturation / S.J. Hong, S.K. Kim, E.B. Ko et al. // Mol. Immunol. — 2017 Jan. — 81. — 135-142. — doi: 10.1016/j.molimm.2016.12.008.
15. Jin J.O. BDCA1-positive dendritic cells (DCs) represent a unique human myeloid DC subset that induces innate and adaptive immune responses to Staphylococcus aureus Infection / J.O. Jin, W. Zhang, J.Y. Du, Q. Yu // Infect. Immun. — 2014 Nov. — 82(11). — 4466-76. — doi: 10.1128/IAI.01851-14.
16. Kamenyeva O. Neutrophil recruitment to lymph nodes limits local humoral response to Staphylococcus aureus / O. Kamenyeva, C. Boularan, J. Kabat et al. // PLoS Pathog. — 2015, Apr 17. — 11(4). — 1004827. — doi: 10.1371/journal.ppat.1004827.
17. Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation // Nat. Rev. Immunol. — 2013 Mar. — 13(3). — 159-75. — doi: 10.1038/nri3399.
18. Kurokawa K., Takahashi K., Lee B.L. The staphylococcal surface-glycopolymer wall teichoic acid (WTA) is crucial for complement activation and immunological defense against Staphylococcus aureus infection // Immunobiology. — 2016 Oct. — 221(10). — 1091-101. — doi: 10.1016/j.imbio.2016.06.003.
19. Lund L.D., Ingmer H., Frøkiær H. D-Alanylation of Teichoic Acids and Loss of Poly-N-Acetyl Glucosamine in Staphylococcus aureus during Exponential Growth Phase Enhance IL-12 Production in Murine Dendritic Cells // PLoS One. — 2016, Feb 12. — 11(2). — 0149092. — doi: 10.1371/journal.pone.0149092.
20. McGuinness W.A., Kobayashi S.D., DeLeo F.R. Evasion of Neutrophil Killing by Staphylococcus aureus // Pathogens. — 2016, Mar 17. — 5(1). — 32. — doi: 10.3390/pathogens5010032.
21. Michea P. Epithelial control of the human pDC response to extracellular bacteria / P. Michea, P. Vargas, M.H. Donnadieu et al. // Eur. J. Immunol. — 2013 May. — 43(5). — 1264-73. — doi: 10. 1002/eji.201242990.
22. Navegantes K.C. Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity / K.C. Navegantes, R. de Souza Gomes, P.A. Pereira et al. // J. Transl. Med. — 2017, Feb 15. — 15(1). — 36. — doi: 10.1186/s12967-017-1141-8.
23. Nguyen Q.T. Role of Interleukin-12 in Protection against Pulmonary Infection with Methicillin-Resistant Staphylococcus aureus / Q.T. Nguyen, Y. Furuya, S. Roberts, D.W. Metzger // Antimicrob. Agents Chemother. — 2015 Oct. — 59(10). — 6308-16. — doi: 10.1128/AAC.00968-15.
24. Nordenfelt P., Tapper H. Phagosome dynamics during phagocytosis by neutrophils // J. Leukoc. Biol. — 2011 Aug. — 90(2). — 271-84. — doi: 10.1189/jlb.0810457.
25. Parcina M. Pathogen-triggered activation of plasmacytoid dendritic cells induces IL-10-producing B cells in response to Staphylococcus aureus / M. Parcina, M.A. Miranda-Garcia, S. Durlanik et al. // J. Immunol. — 2013, Feb 15. — 190(4). — 1591-602. — doi: 10.4049/jimmunol.1201222.
26. Parker D. Innate Immune Signaling Activated by MDR Bacteria in the Airway / D. Parker, D. Ahn, T. Cohen, A. Prince // Physiol. Rev. — 2016 Jan. — 96(1). — 19-53. — doi: 10.1152/physrev.00009.2015.
27. Patel V.I., Metcalf J.P. Identification and characterization of human dendritic cell subsets in the steady state: a review of our current knowledge // J. Investig. Med. — 2016 Apr. — 64(4). — 833-47. — doi: 10.1136/jim-2016-000072.
28. Pilsczek F.H. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus / F.H. Pilsczek, D. Salina, K.K. Poon et al. // J. Immunol. — 2010, Dec 15. — 185(12). — 7413-25. — doi: 10.4049/jimmunol.1000675.
29. Puga I. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen / I. Puga, M. Cols, C.M. Barra et al. // Nat. Immunol. — 2011, Dec 25. — 13(2). — 170-80. — doi: 10.1038/ni.2194.
30. Rankin S.M. The bone marrow: a site of neutrophil clea–rance // J. Leukoc. Biol. — 2010 Aug. — 88(2). — 241-51. — doi: 10.1189/jlb.0210112.
31. Rigby K.M., DeLeo F.R. Neutrophils in innate host defense against Staphylococcus aureus infections // Semin. Immunopathol. — 2012 Mar. — 34(2). — 237-59. — doi: 10.1007/s00281-011-0295-3.
32. Robertson C.M. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance / C.M. Robertson, E.E. Perrone, K.W. McConnell et al. // J. Surg. Res. — 2008 Dec. — 150(2). — 278-85. — doi: 10.1016/j.jss.2008.02.009.
33. Rönnberg E. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo / E. Rönnberg, C.F. Johnzon, G. Calounova et al. // Immunology. — 2014 Oct. — 143(2). — 155-63. — doi: 10.1111/imm.12297.
34. Roquilly A. CpG-ODN and MPLA prevent mortality in a murine model of post-hemorrhage-Staphyloccocus aureus pneumonia / A. Roquilly, L. Gautreau, J.P. Segain et al. // PLoS One. — 2010, Oct 7. — 5(10). — 13228. — doi: 10.1371/journal.pone.0013228.
35. Sadik C.D., Kim N.D., Luster A.D. Neutrophils casca–ding their way to inflammation // Trends Immunol. — 2011 Oct. — 32(10). — 452-60. — doi: 10.1016/j.it.2011.06.008.
36. Scapini P. Human neutrophils in the saga of cellular heterogeneity: insights and open questions / P. Scapini, O. Marini, C. Tecchio, M.A. Cassatella // Immunol. Rev. — 2016 Sep. — 273(1). — 48-60. — doi: 10.1111/imr.12448.
37. Schindler D. Dendritic cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection / D. Schindler, M.G. Gutierrez, A. Beineke et al. // Am. J. Pathol. — 2012 Oct. — 181(4). — 1327-37. — doi: 10.1016/j.ajpath.2012.06.039.
38. Selders G.S. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration / G.S. Selders, A.E. Fetz, M.Z. Radic, G.L. Bowlin // Regen. Biomater. — 2017 Feb. — 4(1). — 55-68. — doi: 10.1093/rb/rbw041.
39. Spaan A.N. Neutrophils versus Staphylococcus aureus: a biological tug of war / A.N. Spaan, B.G. Surewaard, R. Nijland, J.A. van Strijp // Ann. Rev. Microbiol. — 2013. — 67. — 629-50. — doi: 10.1146/annurev-micro-092412-155746.
40. Strydom N., Rankin S.M. Regulation of circulating neutrophil numbers under homeostasis and in disease // J. Innate Immun. — 2013. — 5(4). — 304-14. — doi: 10.1159/000350282.
41. Swiecki M., Colonna M. The multifaceted biology of plasmacytoid dendritic cells // Nat. Rev. Immunol. — 2015 Aug. — 15(8). — 471-85. — doi: 10.1038/nri3865.
42. Thammavongsa V. Staphylococcal manipulation of host immune responses / V. Thammavongsa, H.K. Kim, D. Missiakas, O. Schneewind // Nat. Rev. Microbiol. — 2015 Sep. — 13(9). — 529-43. — doi: 10.1038/nrmicro3521.
43. Thurlow L.R. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo / L.R. Thurlow, M.L. Hanke, T. Fritz et al. // J. Immunol. — 2011, Jun 1. — 186(11). — 6585-96. — doi: 10.4049/jimmunol.1002794.
44. Tong S.Y. Staphylococcus aureus infections: epidemio–logy, pathophysiology, clinical manifestations, and management / S.Y. Tong, J.S. Davis, E. Eichenberger et al. // Clin. Microbiol. Rev. — 2015 Jul. — 28(3). — 603-61. — doi: 10.1128/CMR.00134-14.
45. Tsuda Y. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphy–lococcus aureus / Y. Tsuda, H. Takahashi, M. Kobayashi et al. // Immunity. — 2004 Aug. — 21(2). — 215-26. — doi: 10.1016/j.immuni.2004.07.006.
46. van Kessel K.P., Bestebroer J., van Strijp J.A. Neutrophil-Mediated Phagocytosis of Staphylococcus aureus // Front. Immunol. — 2014, Sep 26. — 5. — 467. — doi: 10.3389/fimmu.2014.00467.
47. Waters E.M. Convergence of Staphylococcus aureus Persister and Biofilm Research: Can Biofilms Be Defined as Communities of Adherent Persister Cells? / E.M. Waters, S.E. Rowe, J.P. O’Gara, B.P. Conlon // PLoS Pathog. — 2016, Dec 29. — 12(12). — 1006012. — doi: 10.1371/journal.ppat.1006012.
48. Worbs T. Dendritic cell migration in health and disease / T. Worbs, S.I. Hammerschmidt, R. Förster et al. // Nat. Rev. Immunol. — 2017 Jan. — 17(1). — 30-48. — doi: 10.1038/nri.2016.116.
49. Wu X., Xu F. Dendritic cells during Staphylococcus aureus infection: subsets and roles // J. Transl. Med. — 2014, Dec 18. — 12. — 358. — doi: 10.1186/s12967-014-0358-z.
50. Yang F. The Diverse Biological Functions of Neutrophils, Beyond the Defense Against Infections / F. Yang, C. Feng, X. Zhang, J. Lu, Y. Zhao // Inflammation. — 2017 Feb. — 40(1). — 311-323. — doi: 10.1007/s10753-016-0458-4.

/168-1.jpg )
/169-1.jpg )
/170-1.jpg )
/171-1.jpg )
/172-1.jpg )
/173-1.jpg )
/175-1.jpg )
/174-1.jpg )