Врожденные лимфоидные клетки
Julia Svedova и соавт. [71] продемонстрировали, что ингаляционное введение энтеротоксина SEA бактерий Staphylococcus aureus нокаутным мышам Tcrβ–/– не сопровождалось высвобождением моноцитов и нейтрофилов в кровь и их рекрутированием в лимфатические узлы до тех пор, пока не было произведено быстрой мобилизации врожденных лимфоидных клеток. Врожденные лимфоидные клетки (innate lymphoid cells — ILC) предотвращают бактериальную транслокацию за пределы эпителиального барьера, индуцируют продукцию IgA в слизистых оболочках и действуют как регуляторы активности иммунной системы. Интересно, что комменсальные бактерии вызывают пролиферацию ILC. Морфологически ILC-клетки подобны лимфоцитам и продуцируют высокие уровни, в зависимости от типа, Th1-, Th2 - и Th17-ассоциированных цитокинов. В настоящее время различают три типа ILC: ILC1, ILC2 и ILC3 (табл. 1) [56, 63].
/144-1.jpg)
/145-1.jpg)
Человеческие ILC1-клетки представлены тремя субпопуляциями: NK-клетками, ILC1-клетками с низкой или отсутствующей экспрессией CD127 (CD127–) и ILC1-клетками с высокой экспрессией CD127 (CD127+). В легочной ткани фенотип клеток CD127–ILC1 характеризуется сигнатурами CD3–CD56+NKp44+CD103+ (клетки с интраэпителиальной локализацией) и CD3–CD56+NKp44–CD103–. Клетки CD127+ILC1 характеризуются фенотипом Lin–CD127+CRTH2+CD117–NKp44–. Данные клетки экспрессируют T-bet, но не экспрессируют хемокиновый рецептор CCR6, CD103 или CD25 [13, 17, 38].
Натуральные киллеры, краткая характеристика которых представлена в табл. 2, при бактериальных инфекциях осуществляют киллинг инфицированных клеток.
/146-1.jpg)
Maren von Köckritz-Blickwede и соавт. [78] продемонстрировали, что NK-клетки практически не влияют на течение инфекционного процесса, вызванного бактериями Staphylococcus aureus. Однако, согласно результатам исследования Cherrie-Lee Small и соавт. [70], при стафилококковой пневмонии не только значительно увеличивается представительство NK-клеток в очаге поражения, но и NK-клетки мобилизуются в люмен респираторного тракта. Мыши, обедненные NK-клетками, более восприимчивы к бактериям Staphylococcus aureus, чем мыши дикого типа. По мнению Hui Zhao и соавт. [83], снижение представительства NK-клеток в ткани легкого, как и ингибиция фагоцитоза у альвеолярных макрофагов, является механизмом, лежащим в основе повышенной восприимчивости к бактериям Staphylococcus aureus и развития стафилококковой пневмонии. Авторы продемонстрировали достоверный приток NK1.1+CD3 NK-клеток в просвет респираторного тракта крыс после заражения бактериями Staphylococcus aureus и способность NK-клеток индуцировать активность фагоцитоза у альвеолярных макрофагов во время пневмонии, вызванной бактериями Staphylococcus aureus. Роль NK-клеток в патогенезе стафилококковой пневмонии представлена на рис. 1.
/149-1.jpg)
Клетки ILC2 идентифицированы в легких плода и взрослых, а также в бронхоальвеолярной лаважной жидкости человека. Фенотип данных клеток в респираторном тракте характеризуется сигнатурой Lin–CD127+CD161+CRTH2+. Маркер CRHT2 специфичен для человека, мышиные ILC2 его не экспрессируют. Клетки ILC2 также экспрессируют ICOS, CD25 и ST2. Среди ILC2 различают две субпопуляции клеток: с высокой и низкой экспрессией KLRG1 [3, 12].
Клетки ILC3 кластеризированы на две основные группы: фетальные клетки LTi и постнатальные (взрослые) ILC3. Фетальные клетки LTi, прототипные ILC3, идентифицируют как CD127+CD3–CD4+-клетки. Человеческие фетальные LTi-клетки впервые были обнаружены в 2009 году как клетки с фенотипом Lin–RORγt+CD127+CD4–. Постнатальные ILC3 определены как CD45+Lin–Thy1+RORγt+-клетки, которые также частично экспрессируют CCR6 и NKp46. Постнатальные ILC3 являются наиболее гетерогенной популяцией ILC. В зависимости от уровня экспрессированного NK-рецептора, например NKp44, NKp46 или NKp30, ILC3 разделены на NCR–ILC3 и NCR+ILC3. В легких человека ILC3 идентифицированы как клетки с фенотипом Lin–CD127+CRTH2–CD117+ и являются NCR– или NCR+ [13, 55].
Клетки ILC1 преимущественно продуцируют IFN-γ; ILC2 в ответ на влияние IL-25 и IL-33 высвобождают Th2-ассоциированные цитокины –(IL-5, IL-9 и IL-13); ILC3 продуцируют IL-17 и IL-22. Считают, что ILC1-клетки в основном предопределяют развитие IFN-γ-опосредованного воспаления респираторного и пищеварительного тракта; ILC2-клетки, продуцируя Th2-ассоциированные цитокины, участвуют в патогенезе аллергических заболеваний респираторного тракта; ILC3-клетки, секретируя IL-22, играют ключевую роль в развитии псориаза [5, 49].
Jonathan S. Silver и соавт. [68, 69] показали, что клетки ILC1, продуцирующие большое количество IFN-γ, участвуют в защите макроорганизма от внутри- и внеклеточных патогенов, в том числе и от бактерий Staphylococcus aureus. Развитие инфекционного процесса сопровождается увеличением представительства T-bet+ILC1 в очаге поражения легких и повышением уровня экспрессии ими рецепторов IL-12R и IL-18R (IL-12Rβ2 и IL-18Rα). В сочетании с увеличением количества ILC1 происходит быстрое (в течение двух дней после заражения) снижение представительства резидентных ILC2-клеток и уровня экспрессии фактора транскрипции GATA3. У ILC2-клеток наблюдается уменьшение экспрессии ST2, CD25 (IL-2Rα), IL-7Rα, индуцибельного костимулятора (ICOS) и рецептора фактора стволовых клеток c-kit (CD117). Представляет интерес тот факт, что снижение экспрессии GATA3 отрицательно коррелирует с повышением уровня IL-18Rα. Авторы считают, что при инфицировании легкого ILC2-клетки мигрируют в очаг поражения и под воздействием провоспалительных цитокинов IL-12 и IL-18, продуцируемых DC и макрофагами, преобразуются в ILC1-клетки.
Клетки ILC3 играют важную роль в поддержании барьерной функции слизистой оболочки. Во время бактериальной пневмонии цитокины IL-1β и IL-23, продуцируемые PAMP-индуцированными DC и макрофагами, активируют пульмональные ILC3. Активированные ILC3 высвобождают IL-17, IL-22, IL-8/СХСL8, IL-2, TNF-α и лимфотоксин LTα1β2 (lymphotoxin-alpha 1 beta 2). Цитокины IL-22 и IL-17 активируют эпителиоциты и вызывают у них секрецию антимикробных пептидов [2, 76]. Также IL-17 индуцирует продукцию IL-12 DC, ингибирует IL-10, усиливает цитодифференцировку наивных Т-лимфоцитов в Th17-клетки. Пульмональные резидентные Th17-клетки индуцируют секрецию лигандов хемокинового рецептора CXCR3, рекрутируя Th1-клетки во время инфекционно-воспалительного процесса. Цитокин IL-22 участвует в репарации эпителия респираторного тракта [52]. Цитокины IL-2 и IL-8/СХСL8 вербуют нейтрофилы в очаг поражения легкого. Лимфотоксин LTα1β2 стимулирует экспрессию ICAM-1 и VCAM-1 мезенхимальными стволовыми клетками [1, 16].
Возможная роль врожденных лимфоидных клеток в легочной ткани во время стафилококковой инфекции представлена на рис. 2.
Т-лимфоциты
Активация и пролиферация Т-клеток считается основным компонентом воспалительного процесса, вызванного энтеротоксинами бактерий Staphylococcus aureus [27]. Установлено, что во время острой стафилококковой инфекции после контакта с бактериями происходит активная пролиферация Т-клеток, а при хронической инфекции данный ответ полностью отсутствует. Однако во время хронической стафилококковой инфекции значение вклада Т-клеток в элиминацию бактерий Staphylococcus aureus существенно снижается [84]. Продемонстрировано, что у экспериментальных животных, дефицитных по Т-, В- и NK-клеткам, не отмечается критического дефекта элиминации бактерий Staphylococcus aureus во время хронического течения заболевания, а Т-клетки оказываются необязательным компонентом бактериального клиренса при стафилококковой инфекции [78]. Глубокая супрессия T-клеток при хронической стафилококковой инфекции обусловлена преимущественно влиянием клеток-супрессоров миелоидного происхождения (myeloid-derived suppressor cells — MDSC) и незначительным действием Treg-клеток [72].
Т-лимфоциты характеризуются наличием на поверхности мембраны Т-клеточного рецептора (T cell receptors — TCR), который состоит из двух цепей: чаще всего из α- и β- или из γ- и δ-цепей, в связи с чем различают αβТ- и γδТ-клетки [4]. Среди αβТ-клеток выделяют клетки, характеризующиеся экспрессией инвариантной или полуинвариантной α-цепи TCR: инвариантные ассоциированные Т-клетки, связанные со слизистыми оболочками (mucosal-associated invariant T cells — MAIT), инвариантные натуральные киллерные Т-клетки (invariant natural killer T cells — iNKT). Инвариантные αβТ-клетки и γδТ-клетки представляют популяцию врожденных Т-лимфоцитов, быстро реагирующих на инфицирование, — активация данных клеток занимает менее двух часов (табл. 3) [37].
Т-клетки врожденной иммунной системы
αβТ-клетки
MAIT-клетки
Отличительной чертой MAIT-клеток человека является их способность экспрессировать полуинвариантный рецептор TCR, который содержит инвариантную α-цепь TRAV1-2-TRAJ33 (Vα7.2-Jα33), преимущественно ассоциированную с β-цепями TRBV20 (Vβ2) или TRBV6 (Vβ13) [35].
Фенотипически MAIT-клетки характеризуются сигнатурой CD3+Vα7.2+CD161++ и либо CD8+, либо дважды отрицательным маркером (CD4–CD8–). Также MAIT-клетки коэкспрессируют IL-18R и CD26. В периферической крови взрослых MAIT-клетки приобретают фенотип клеток эффекторной памяти (CD45RO+CD62LloCD95hiCD122intCD127int) и экспрессируют хемокиновые рецепторы (CCR5, CCR6, CXCR6 и CCR9). Необходимо отметить, что MAIT-клетки не экспрессируют CCR7, который является маркером для возвращения клеток в лимфатические узлы [26]. Клетки данной субпопуляции Т-лимфоцитов в основном расположены в слизистых оболочках и периферической крови, могут находиться в тканях легких, кишечника и печени. У людей доля MAIT-клеток составляет от 1 до 10 % общей популяции CD3+Т-клеток, циркулирующих в периферическом русле крови [12, 82]. У пациентов с пневмонией во время фазы разгара значительно уменьшается присутствие MAIT-клеток в периферическом русле крови [48]. По мнению Anda Meierovics и соавт. [53], причина снижения содержания MAIT-клеток в периферическом русле крови связана с миграцией данных клеток в инфицированный очаг легкого.
Все человеческие MAIT-клетки экспрессируют фактор транскрипции — протеин цинкового пальца промиелоцитарного лейкоза PLZF (promyelocytic leukemia zinc finger) [61].
Активация клеток MAIT может быть результатом либо TCR-зависимого возбуждения (инициируемого лигандом, который представлен MR1-антиген–презентирующей клеткой), либо TCR-независимого возбуждения [82].
Рецептор TCR MAIT-клеток распознает антигены, которые презентируются высококонсервативной мономорфной молекулой MR1 (MHC class I-related protein) главного комплекса гистосовместимости (MHC). В настоящее время установлено, что метаболиты незаменимого витамина B2 (рибофлавина) являются MR1-зависимыми лигандами для TCR MAIT-клеток. Рибофлавиновые антигены, активирующие MAIT-клетки, генерируются патогенными и комменсальными бактериями [24], TCR-независимым способом MAIT-клетки активируются при помощи цитокинов IL-1β, IL-12, IL-18, IL-23 [26].
Активированные MAIT-клетки участвуют в инфекционном процессе, продуцируя провоспалительные цитокины, активируя иммуноциты и лизируя зараженные клетки.
В основном MAIT-клетки продуцируют такие цитокины, как IFN-γ, TNF-α, IL-2, IL-17A, но не секретируют IL-10 [18].
Продемонстрировано, что MAIT-клетки способствуют ранней продукции GM-CSF, который индуцирует дифференцировку CCR2-зависимых моноцитов в moDC в легких во время пневмонии, вызванной Francisella tularensis LVS [54].
Киллинг инфицированных клеток MAIT-клетки осуществляют посредством гранзим-перфоринового механизма. Необходимо подчеркнуть, что человеческие MAIT-клетки отличаются уникальным цитотоксическим профилем, который характеризуется низким уровнем экспрессии перфорина в сочетании с высоким уровнем экспрессии гранзимов A и K [45]. Установлено, что MAIT-клетки способны лизировать эпителиальные клетки, инфицированные бактериями [47].
Установлено, что MAIT-клетки принимают активное участие в патогенезе острых инфекций респираторного тракта. Так, показана инфильтрация легочной ткани MAIT-клетками в острый период и период разгара при пневмонии, вызванной Francisella tularensis [54]. У мышей с нокаутным геном Mr1 наблюдается снижение бактериального клиренса ткани легких при бактериальных пневмониях, в частности вызванных Francisella tularensis и Klebsiella pneumoniae [29, 53]. Согласно мнению Timothy S.C. Hinks [35], на ранних стадиях ответа иммунной системы во время инфекционного процесса эффекты, ассоциированные с функционированием MAIT-клеток, могут заметно превалировать над проявлениями реакций конвенциональных пептид-специфичных αβT-клеток.
Maria A. Johansson и соавт. [39] установили, что компоненты клеточной стенки бактерий Staphylococcus aureus активируют MAIT-клетки в дополнение к CD4+ и CD8+Т-клеткам. Энтеротоксины, продуцируемые бактериями Staphylococcus aureus, классически считаются индукторами поликлональной активации адаптивных Т-клеток. Однако показано, что стафилококковый энтеротоксин SEA индуцирует экспрессию IFN-γ и в MAIT-клетках. Вероятная роль MAIT-клеток при развитии стафилококковой пневмонии представлена на рис. 3.
iNKT-клетки
Клетки iNKT представляют собой клетки памяти, несущие одновременно рецепторы как TCR, так и KLRB1 (killer cell lectin like receptor B1/CD161), характерные для Т-лимфоцитов и NK-клеток соответственно. Клетки iNKT несут полуинвариантный TCR, используя который они распознают липидные антигены — как собственные, так и бактериальные. Функционально инвариантные натуральные киллерные Т-клетки связывают врожденные и адаптивные реакции иммунной системы. Во время инфекционного процесса iNKT-клетки индуцируют матурацию DC [19, 65].
Среди CD1d-рестриктированных iNKT-клеток в зависимости от репертуара TCR и антигенного профиля различают два типа лимфоцитов (табл. 4) [9].
Также идентифицированы NKTFH (фолликулярные хелперные клетки), NKT10 и Foxp3+ iNKT-клетки. Фолликулярные хелперные клетки NKTFH дифференцируются после взаимодействия NKT-клеток с В-клетками при инфекционном процессе и индуцируют устойчивый гуморальный ответ [62]. Клетки NKT10 конститутивно продуцируют IL-10, экспрессию которого регулирует E-протеин E2A, возможно, совместно с IRF4 [81]. В условиях экспериментального аутоиммунного энцефаломиелита стимулирование α-GalCer приводит к формированию в дренирующих ЦНС лимфатических узлах iNKT клеток, экспрессирующих фактор транскрипции Foxp3. Более того, как человеческие, так и мышиные iNKT-клетки, in vitro стимулированные TGF-β, могут экспрессировать Foxp3 [11].
Клетки iNKT I типа человека экспрессируют TCR, в котором инвариантная α-цепь (Vα24-Jα18) сочетается с ограниченным репертуаром Vβ-цепи (Vβ8.2, Vβ7). Ключевым отличием iNKT I типа является раннее и быстрое высвобождение цитокинов и хемокинов. Клетки iNKT II типа экспрессируют более разнообразный репертуар TCR и распознают различные липиды, ассоциированные с CD1d (табл. 5) [44, 64].
Клетки iNKT у человека представляют собой небольшую Т-клеточную субпопуляцию, которая составляет примерно 0,01–0,1 % периферических Т-клеток [6]. В ткани легких неактивные iNKT-клетки располагаются в крови микроциркуляторного русла, а после активации перемещаются в паренхиму легких [66].
iNKT-клетки могут быть активированы TCR-зависимым и TCR-независимым способом.
Клетки iNKT распознают липидные антигены, в большинстве случаев гликолипиды, которые презентированы молекулой CD1d. Первым клеточным антигеном, распознаваемым iNKT-клетками, был идентифицирован агеласфин (agelasphin) 9b, выделенный из морской губки Agelas mauritanius. Наиболее известным лигандом TCR iNKT-клеток является α-галактозилцерамид (α-galactosylceramide — αGalCer), чувствительность к которому отличает iNKT-клетки I и II типов [7, 12].
Цитокины врожденных иммунных клеток вместе с сигналами TCR содействуют индукции секреции цитокинов из iNKT-клеток. Учитывая, что клетки iNKT экспрессируют рецепторы для различных цитокинов, включая IL-1β, IL-12, IL-18, IL-23, IL-25 и IL-33, они могут быть ими активированы. Различные субпопуляции iNKT-клеток отличаются по уровню представительства цитокиновых рецепторов на поверхности своей мембраны. Так, NKT1-клетки преимущественно экспрессируют IL-12R; NKT2-клетки — IL-25R, IL-17RB; NKT17-клетки — IL-1R и IL-23R. Таким образом, iNKT-клетки могут быть активированы TLR-индуцированными цитокинами, продуцируемыми различными иммуноцитами [11, 43].
После активации рецептора TCR клетки iNKT начинают продуцировать провоспалительные цитокины и могут проявлять цитотоксическую активность. Так, iNKT-клетки I типа продуцируют Th1- и Th2-ассоциированные цитокины: NKT1 высоко экспрессируют фактор транскрипции T-bet и продуцируют IFNγ, IL-13, IL-4; NKT2-клетки продуцируют IL-4 и IL-13; а NKT17-клетки — IL-17A [12]. Клетки NKT I и II типа активируют антигенпрезентирующие клетки, в том числе DC и B-лимфоциты. Однако клетки NKT II типа возбуждают pDC и оказывают толерогенный эффект на сDC [21].
Согласно результатам экспериментальных исследований, инфекционный процесс, вызванный бактериями Staphylococcus aureus, сопровождается активацией iNKT-клеток [10]. Установлено, что iNKT-клетки могут быть активированы негликосфинголипидными эндогенными антигенами. Jacqueline L. Hayworth и соавт. [33] продемонстрировали, что активация iNKT-клеток энтеротоксином SEB бактерий Staphylococcus aureus в отличие от стимуляции iNKT-клеток α-галактозилцерамидом не сопровождается возбуждением TCR-ассоциированных сигнальных путей. Авторы считают, что бактериальный энтеротоксин SEB, нацеливающийся на Vβ8 цепь, возбуждает iNKT-клетки, используя новый путь, который требует взаимодействия с молекулами МНС II класса, но не с молекулой CD1d.
Продемонстрировано, что мыши, лишенные iNKT-клеток I типа, отличаются более низкими уровнями циркулирующих в периферическом русле крови IFN-γ и TNF-α и более высокой степенью выживаемости при индукции системной реакции Шварцмана — Санарелли [22]. Также установлено, что у мышей C57BL/6J с дефицитом Jα18, не имеющих iNKT-клеток I типа, наблюдается более низкий уровень провоспалительных цитокинов в сыворотке крови и более высокая степень выживаемости при экспериментальном полимикробном септическом шоке, чем у мышей дикого типа [36]. Установлено, что iNKT-клетки принимают непосредственное участие в патогенезе пневмоний, вызванных как грамположительными, так и грамотрицательными бактериями [28].
Возможная роль iNKT-клеток в патогенезе стафилококковой пневмонии представлена на рис. 4.
Jakub Kwiecinski и соавт. [46] показали, что, несмотря на активацию iNKT-клеток I типа бактериями Staphylococcus aureus, не существует достоверной разницы в уровне смертности у мышей, лишенных NKT-клеток I типа (Jα18–/–), или у CD1d-дефицитных мышей по сравнению с мышами дикого типа. Согласно мнению авторов, основанному на результатах данного исследования модели стафилококкового сепсиса, клетки I типа iNKT могут стимулировать развитие эндотоксического шока и полимикробного сепсиса, но iNKT-клетки ни I, ни II типа не играют существенной роли в танатогенезе при стафилококковой инвазивной инфекции.
γδT-клетки
Существующие только у приматов γδT-клетки в отличие от αβT-клеток не экспрессируют маркеры CD4 или CD8 и не требуют презентации антигена молекулами MHC [40, 61].
В зависимости от строения TCR γδT-клетки образуют несколько субпопуляций, для которых характерна конкретная локализация пребывания. Так, большинство человеческих γδT-клеток в крови (2–10 % периферических Т-клеток) несут на мембране Vγ9Vδ2TCR, а γδT-клетки эпителия и слизистых оболочек — Vδ1Vδ3TCR. В респираторном тракте γδT-клетки представляют малочисленную субпопуляцию, которая равномерно распределена в паренхиматозных и непаренхиматозных регионах легкого [79].
Активация γδT-клеток может осуществляться как зависимым, так и не зависимым от TCR способом, который ассоциирован с возбуждением TLR, KLRK1, лектиновых, интерлейкиновых и других рецепторов [8]. Спектр антигенов, распознаваемых γδTCR, достаточно широк: человеческие Vγ9Vδ2Т-клетки реагируют с разнообразными продуктами мевалонатного пути [30]. Vγ9Vδ2T-лимфоциты являются основной субпопуляцией γδT-клеток, которая определяет уровень антибактериальной защиты. Данные лимфоциты в присутствии антигенпрезентирующих клеток независимо от MHC участвуют в рекогниции фосфорилированных метаболитов пренила, например (E)-4-гидрокси-3-– метилбут-2-енил пирофосфата ((E)-4-hydroxy-3-methyl-but-2-enyl pyrophsphate — HMBPP). Уровень концентрации пренила высоко коррелирует со степенью активации и пролиферации Vγ9Vδ2Т-клеток [62]. Решающую роль в активации Vγ9Vδ2T-клеток играет бутирофилин-3 BTN3A1 (CD277), который связывается с бактериальными фосфорилированными антигенами, в частности с HMBPP, а рецептор Vγ9Vδ2TCR взаимодействует с комплексом BTN3A1-антиген [32, 77, 80]. Vδ1+Т-клетки распознают липиды, представленные молекулами CD1 [75], не процессированные протеины, в том числе вирусные белки, фикоэритрин, инсулиновый пептид и индуцированные стрессом молекулы [37].
γδT-клетки способствуют бактериальному клиренсу, непосредственно продуцируя антимикробные пептиды LL-37, элафин, дефензины [23, 50]. γδT-клетки продуцируют широкий спектр цитокинов, в том числе IL-17 или IFN-γ, TNF-α, и проявляют сильную цитотоксическую активность против инфицированных или трансформированных клеток, используя цитолитические протеины (перфорин и гранзим) [20, 31, 57].
Возбуждение γδT-клеток характеризуется дифференцированным ответом в зависимости от активированного рецептора. Так, активация рецептора γδTCR сопровождается продукцией IFN-γ, TNF-α, CCL3, CCL4, CCL5; активация костимуляторных молекул CD27 и CD30 индуцирует увеличение внутриклеточной концентрации ионов кальция, что приводит к секреции IL-4 и IFN-γ; возбуждение Notch вызывает продукцию IL-17, а Skint-1 (selection and upkeep of intraepithelial T cells 1) — IFN-γ; активация рецептора KLRK1 индуцирует секрецию гранзимов и перфорина; а TLR — провоспалительных цитокинов и хемокинов [60].
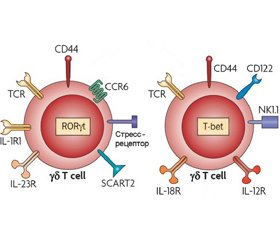
Считают, что δT-клетки являются основными продуцентами провоспалительных цитокинов: IFN-γ и IL-17. Продукция данных цитокинов зависит от активации рецептора γδTCR: γδT-клетки с активированным TCR преимущественно секретируют IFN-γ, а γδT-клетки с неактивированным или слабо активированным TCR продуцируют IL-17. Таким образом, наивные γδT-клетки продуцируют IL-17, а антигениндуцированные γδT-клетки — IFN-γ (рис. 5) [20].
/151-1.jpg)
Показано, что активация TCR возбуждает фактор раннего ответа 3 (early growth response 3 — EGR3), что, в свою очередь, подавляет экспрессию фактора транскрипции RORγt и, как следствие, продукцию IL-17 γδT-клетками [73]. В то же время фактор Egr3 способствует пролиферации γδT-клеток и продукции IL-17 [59]. Rose M. Parkinson и соавт. [59] продемонстрировали, что у мышей с избыточной экспрессией Egr3 наблюдается пятикратное увеличение количества γδТ-клеток в ткани легких и селезенки по сравнению с мышами дикого типа, что подчеркивает значение фактора транскрипции Egr3 в поддержании популяции γδT-клеток. У мышей с избыточной экспрессией Egr3 отмечается высокий уровень активности воспаления и степени фиброза легких. Трансгенные мыши отличаются более низкой выживаемостью от мышей дикого типа, экспрессирующих нормальные уровни Egr3. Авторы считают, что IL-17-продуцирующие γδТ-клетки в легких выполняют ключевую провоспалительную роль, однако чрезмерная активация данных клеток может привести к неблагоприятному течению воспаления.
Известно, что γδT-клетки играют существенную роль в раннем периоде иммунного ответа на патогенные микроорганизмы, так как γδT-клетки рекрутируют нейтрофилы, DC и макрофаги [42, 51, 58].
Через 6 часов после инфицирования респираторного тракта бактериями Staphylococcus aureus в ткани легкого абсолютное количество γδТ-клеток увеличивается в 6 раз. Увеличение представительства γδT-клеток в пневмоническом очаге обусловлено пролиферацией резидентных пульмональных γδT-клеток и рекрутированием из периферической крови циркулирующих γδT-клеток в ткань легкого [15].
Истощение γδТ-клеток приводит к нарушению защиты макроорганизма от бактерий Streptococcus pneumoniae [41] и Klebsiella pneumonia [74].
Установлено, что интраназальная инстилляция сублетальной дозы (5 × 108 КОЕ) бактерий Staphylococcus aureus сопровождается у мутантных гомозиготных мышей с делецией гена Tcr (Tcrδ–/–) более высокой бактериальной нагрузкой в ткани легких и селезенки через 24 и 48 часов после инфицирования, чем у мышей дикого типа. Вероятно, нарушение бактериального клиренса обусловлено тем, что отсутствие γδТ-клеток нарушает рекрутирование CD11b+Gr-1high-нейтрофилов в легкие после заражения Staphylococcus aureus [15].
Ping Cheng и соавт. [15] считают, что в ткани легкого γδТ-клетки обеспечивают раннюю продукцию IL-17, который индуцирует экспрессию таких нейтрофилрекрутирующих хемокинов, как CXCL2, CXCL1, во время развития стафилококковой пневмонии. У мышей Tcrδ–/– через 6 ч после инфицирования Staphylococcus aureus наблюдается низкая экспрессия данных хемокинов, а также цитокинов GM-CSF, IL-6 и TNF-α в отличие от мышей дикого типа. Представляет интерес то, что инфицирование мутантных мышей Tcrδ–/– летальной дозой (5 × 109 КОЕ) бактерий Streptococcus pneumonia сопровождается развитием более легкого течения пневмонии, чем у мышей дикого типа. В то же время необходимо отметить, что уровень летальности не зависит от активности γδТ-клеток. Таким образом, отсутствие γδТ-клеток приводит к снижению эффективности бактериального клиренса, но препятствует тяжести поражения легких во время стафилококковой инфекции [15].
Участие γδТ-клеток в патогенезе стафилококковой пневмонии схематически представлено на рис. 6.
Представляет интерес тот факт, что на фоне применения антибиотиков наблюдается сопряженное сокращение не только количества резидентных бактерий, но и представительства γδТ-клеток, продуцирующих IL-17, в ткани легких экспериментальных мышей [14]. Уменьшение представительства γδТ-клеток не зависит от видовой принадлежности подавляемой резидентной флоры. Отсутствие или снижение численности комменсальных бактерий способствует снижению представительства IL-17-продуцирующих γδT-клеток. В результате антибиотикассоциированного подавления активности IL-17-продуцирующих γδT-клеток у мышей увеличивается вероятность развития опухолей легких.
Таким образом, γδТ-клетки в легких, по-видимому, являются активными представителями первой линии неспецифической защиты от инвазивных бактериальных патогенов, в том числе и от бактерий Staphylococcus aureus.
Конфликт интересов. Авторы заявляют об отсутствии какого-либо конфликта интересов при подготовке данной статьи.
Список литературы
1. Ahn Y.O. Human group3 innate lymphoid cells express DR3 and respond to TL1A with enhanced IL-22 production and –IL-2-dependent proliferation / Ahn Y.O., Weeres M.A., Neulen M.L. et al. // Eur. J. Immunol. 2015 Aug; 45(8): 2335-42. doi: 10.1002/eji.201445213.
2. Archer N.K. Interleukin-17A (IL-17A) and IL-17F Are Critical for Antimicrobial Peptide Production and Clearance of Staphylococcus aureus Nasal Colonization / N.K. Archer, N.D. Adappa, J.N. Palmer et al. // Infect. Immun. 2016 Nov 18; 84(12): 3575-3583.
3. Aron J.L., Akbari O. Regulatory T cells and type 2 innate lymphoid cell-dependent asthma // Allergy. 2017 Feb 4. doi: 10.1111/all.13139.
4. Attaf M. The T cell antigen receptor: the Swiss army knife of the immune system / M. Attaf, M. Legut, D.K. Cole, A.K. Sewell // Clin. Exp. Immunol. 2015 Jul; 181(1): 1-18. doi: 10.1111/cei.12622.
5. Bekeredjian-Ding I., Stein C., Uebele J. The Innate Immune Response Against Staphylococcus aureus // Curr. Top. Microbiol. Immunol. 2015 Dec 15. doi: 10.1007/82_2015_5004.
6. Berzins S.P., Smyth M.J., Baxter A.G. Presumed guilty: natural killer T cell defects and human disease // Nat. Rev. Immunol. 2011 Feb; 11(2): 131-42. doi: 10.1038/nri2904.
7. Birkholz A.M., Kronenberg M. Antigen specificity of invariant natural killer T-cells // Biomed. J. 2015 Dec; 38(6): 470-83. doi: 10.1016/j.bj.2016.01.003.
8. Born W.K., Kemal Aydintug M., O’Brien R.L. Diversity of γδ T-cell antigens // Cell. Mol. Immunol. 2013 Jan; 10(1): 13-20. doi: 10.1038/cmi.2012.45.
9. Brennan P.J. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions / P.J. Brennan, M. Brigl, M.B. Brenner et al. // Nat. Rev. Immunol. 2013 Feb; 13(2): 101-17. doi: 10.1038/nri3369.
10. Brigl M., Brenner M.B. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens // Semin. Immunol. 2010 Apr; 22(2): 79-86. doi: 10.1016/j.smim.2009.10.006.
11. Buechel H.M., Stradner M.H., D’Cruz L.M. Stages versus subsets: Invariant Natural Killer T cell lineage differentiation // Cytokine. 2015 Apr; 72(2): 204-9. doi: 10.1016/j.cyto.2014.12.0050.
12. Chandra S., Kronenberg M. Activation and Function of iNKT and MAIT Cells // Adv. Immunol. 2015; 127: 145-201. doi: 10.1016/bs.ai.2015.03.003.
13. Cheng H. Guards at the gate: physiological and pathological roles of tissue-resident innate lymphoid cells in the lung / H. Cheng, C. Jin, J. Wu et al. // Protein Cell. 2017 Mar 7. doi: 10.1007/s13238-017-0379-5.
14. Cheng M. Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism / M. Cheng, L. Qian, G. Shen et al. // Cancer Res. 2014 Aug 1; 74(15): 4030-41. doi: 10.1158/0008-5472.CAN-13-2462.
15. Cheng P. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia / P. Cheng, T. Liu, W.Y. Zhou et al. // BMC Immunol. 2012 Jul 9; 13: 38. doi: 10.1186/1471-2172-13-38.
16. Cording S. Development and regulation of RORγt(+) innate lymphoid cells / S. Cording, J. Medvedovic, M. Cherrier, G. Eberl // FEBS Lett. 2014 Nov 17; 588(22): 4176-81. doi: 10.1016/j.febslet.2014.03.034.
17. Cortez V.S., Colonna M. Diversity and function of group 1 innate lymphoid cells // Immunol. Lett. 2016 Nov; 179: 19-24. doi: 10.1016/j.imlet.2016.07.005.
18. Cowley S.C. MAIT cells and pathogen defense // Cell. Mol. Life Sci. 2014 Dec; 71(24): 4831-40. doi: 10.1007/s00018-014-1708-y.
19. Crosby C.M., Kronenberg M. Invariant natural killer T cells: front line fighters in the war against pathogenic microbes // Immunogenetics. 2016 Aug; 68(8): 639-48. doi: 10.1007/s00251-016-0933-y.
20. Cua D.J., Tato C.M. Innate IL-17-producing cells: the sentinels of the immune system // Nat. Rev. Immunol. 2010 Jul; 10(7): 479-89. doi: 10.1038/nri2800.
21. Dhodapkar M.V., Kumar V. Type II NKT Cells and Their Emerging Role in Health and Disease // J Immunol. 2017 Feb 1; 198(3): 1015-1021. doi: 10.4049/jimmunol.1601399.
22. Dieli F. Resistance of natural killer T cell-deficient mice to systemic Shwartzman reaction / F. Dieli, G. Sireci, D. Russo et al. // J. Exp. Med. 2000 Dec 4; 192(11): 1645-52. PMID: 11104806.
23. Dudal S. Release of LL-37 by activated human Vgamma9Vdelta2 T cells: a microbicidal weapon against Brucella suis / S. Dudal, C. Turriere, S. Bessoles et al. // J. Immunol. 2006 Oct 15; 177(8): 5533-9. doi: 10.4049/jimmunol.177.8.5533.
24. Eckle S.B. Recognition of Vitamin B Precursors and Bypro–ducts by Mucosal Associated Invariant T Cells / S.B. Eckle, A.J. Corbett, A.N. Keller et al. // J. Biol. Chem. 2015 Dec 18; 290(51): 30204-11. doi: 10.1074/jbc.R115.685990.
25. Espinosa E. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells / E. Espinosa, C. Belmant, F. Pont et al. // J. Biol. Chem. 2001 May 25; 276(21): 18337-44. doi: 10.1074/jbc.M100495200.
26. Franciszkiewicz K. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells / K. Franciszkiewicz, M. Salou, F. Legoux et al. // Immunol. Rev. 2016 Jul; 272(1): 120-38. doi: 10.1111/imr.12423.
27. Fraser J.D., Proft T. The bacterial superantigen and superantigen-like proteins // Immunol. Rev. 2008 Oct; 225: 226-43. doi: 10.1111/j.1600-065X.2008.00681.x.
28. Gao Y., Williams A.P. Role of Innate T Cells in Anti-Bacterial Immunity // Front. Immunol. 2015 Jun 11; 6: 302. doi: 10.3389/fimmu.2015.00302.
29. Georgel P. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice / P. Georgel, M. Radosavljevic, C. Macquin, S. Bahram // Mol. Immunol. 2011 Feb; 48(5): 769-75. doi: 10.1016/j.molimm.2010.12.002.
30. Gober H.J. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells / H.J. Gober, M. Kistowska, L. Angman et al. // J. Exp. Med. 2003 Jan 20; 197(2): 163-8. doi: 10.1084/jem.20021500.
31. Hao J. Current progress in γδ T-cell biology / J. Hao, X. Wu, S. Xia et al. // Cell. Mol. Immunol. 2010 Nov; 7(6): 409-13. doi: 10.1038/cmi.2010.50.
32. Harly C. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset / C. Harly, Y. Guillaume, S. Nedellec et al. // Blood. 2012 Sep 13; 120(11): 2269-79. doi: 10.1182/blood-2012-05-430470.
33. Hayworth J.L. CD1d-independent activation of mouse and human iNKT cells by bacterial superantigens / J.L. Hayworth, D.M. Mazzuca, S. Maleki Vareki et al. // Immunol. Cell. Biol. 2012 Aug; 90(7): 699-709. doi: 10.1038/icb.2011.90.
34. Hazenberg M.D., Spits H. Human innate lymphoid cells // Blood. 2014 Jul 31; 124(5): 700-9. doi: 10.1182/blood-2013-11-427781.
35. Hinks T.S. Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease // Immuno–logy. 2016 May; 148(1): 1-12. doi: 10.1111/imm.12582.
36. Hu C.K. The role of hepatic invariant NKT cells in systemic/local inflammation and mortality during polymicrobial septic shock / C.K. Hu, F. Venet, D.S. Heffernan et al. // J. Immunol. 2009 Feb 15; 182(4): 2467-75. doi: 10.4049/jimmunol.0801463.
37. Ivanov S., Paget C., Trottein F. Role of non-conventional T lymphocytes in respiratory infections: the case of the pneumococcus // PLoS Pathog. 2014 Oct 9; 10(10): e1004300. doi: 10.1371/journal.ppat.1004300.
38. Jiao Y. Type 1 Innate Lymphoid Cell Biology: Lessons Learnt from Natural Killer Cells / Y. Jiao, N.D. Huntington, G.T. Belz, C. Seillet // Front. Immunol. 2016 Oct 12; 7: 426. doi: 10.3389/fimmu.2016.00426.
39. Johansson M.A. Probiotic Lactobacilli Modulate Staphylococcus aureus-Induced Activation of Conventional a Unconventional T cells and NK Cells / M.A. Johansson, S. Björkander, M. Mata Forsberg et al. // Front. Immunol. 2016 Jul 11; 7: 273. doi: 10.3389/fimmu.2016.00273.
40. Kalyan S., Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic // Cell. Mol. Immunol. 2013 Jan; 10(1): 21-9. doi: 10.1038/cmi.2012.44.
41. Kirby A.C. Evidence for the involvement of lung-speci–fic gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection / A.C. Kirby, D.J. Newton, S.R. Carding, P.M. Kaye // Eur. J. Immunol. 2007 Dec; 37(12): 3404-13. doi: 10.1002/eji.200737216.
42. Kirby A.C. Pulmonary dendritic cells and alveolar macrophages are regulated by gammadelta T cells during the resolution of S. pneumoniae-induced inflammation / A.C. Kirby, D.J. Newton, S.R. Carding, P.M. Kaye // J. Pathol. 2007 May; 212(1): 29-37. doi: 10.1002/path.2149.
43. Kohlgruber A.C. Activation strategies for invariant natural killer T cells / A.C. Kohlgruber, C.A. Donado, N.M. LaMarche et al. // Immunogenetics. 2016 Aug; 68(8): 649-63. doi: 10.1007/s00251-016-0944-8.
44. Kumar V., Delovitch T.L. Different subsets of natural killer T cells may vary in their roles in health and disease // Immunology. 2014 Jul; 142(3): 321-36. doi: 10.1111/imm.12247.
45. Kurioka A. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets / A. Kurioka, J.E. Ussher, C. Cosgrove et al. // Mucosal. Immunol. 2015 Mar; 8(2): 429-40. doi: 10.1038/mi.2014.81.
46. Kwiecinski J. Sulfatide attenuates experimental Staphylococcus aureus sepsis through a CD1d-dependent pathway / J. Kwiecinski, S. Rhost, L. Löfbom et al. // Infect. Immun. 2013 Apr; 81(4): 1114-20. doi: 10.1128/IAI.01334-12.
47. Le Bourhis L. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells / Le Bourhis L., M. Dusseaux, A. Bohineust et al. // PLoS Pathog. 2013; 9(10): e1003681. doi: 10.1371/journal.ppat.1003681.
48. Le Bourhis L. Antimicrobial activity of mucosal-associated invariant T cells / L. Le Bourhis, E. Martin, I. Péguillet et al. // Nat. Immunol. 2010 Aug; 11(8): 701-8. doi: 10.1038/ni.1890.
49. Marashian S.M. Role of Innate Lymphoid Cells in Lung Dise–ase / S.M. Marashian, E. Mortaz, H.R. Jamaati et al. // Iran J. Allergy Asthma Immunol. 2015 Aug; 14(4): 346-60. PMID: 26547702.
50. Marischen L. Human gammadelta T cells produce the protease inhibitor and antimicrobial peptide elafin / L. Marischen, D. Wesch, J.M. Schröder et al. // Scand. J. Immunol. 2009 Dec; 70(6): 547-52. doi: 10.1111/j.1365-3083.2009.02337.x.
51. Mathews J.A. γδ T Cells Are Required for M2 Macrophage Polarization and Resolution of Ozone-I uced Pulmonary Inflammation in Mice / J.A. Mathews, D.I. Kasahara, L. Ribeiro et al. // PLoS One. 2015 Jul 2; 10(7): e0131236. doi: 10.1371/journal.pone.0131236.
52. McAleer J.P., Kolls J.K. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense // Immunol. Rev. 2014 Jul; 260(1): 129-44. doi: 10.1111/imr.12183.
53. Meierovics A., Yankelevich W.J., Cowley S.C. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection // Proc. Natl. Acad. Sci. USA. 2013 Aug 13; 110(33): E3119-28. doi: 10.1073/pnas.1302799110.
54. Meierovics A.I., Cowley S.C. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection // J. Exp. Med. 2016 Nov 14; 213(12): 2793-2809.
55. Melo-Gonzalez F., Hepworth M.R. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells // Immunology. 2017 Mar; 150(3): 265-275. doi: 10.1111/imm.12697.
56. Mjösberg J., Spits H. Human innate lymphoid cells // J. Allergy Clin. Immunol. 2016 Nov; 138(5): 1265-1276. doi: 10.1016/j.jaci.2016.09.009.
57. Nada M.H. Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation / M.H. Nada, H. Wang, G. Workalemahu, Y. Tanaka, C.T. Morita // J. Immunother. Cancer. 2017 Feb 21; 5: 9. doi: 10.1186/s40425-017-0209-6.
58. Nakasone C. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection / C. Nakasone, N. Yamamoto, M. Nakamatsu et al. // Microbes. Infect. 2007 Mar; 9(3): 251-8. doi: 10.1016/j.micinf.2006.11.015.
59. Parkinson R.M. Egr3 induces a Th17 response by promo–ting the development of γδ T cells / R.M. Parkinson, S.L. Collins, M.R. Horton, J.D. Powell // PLoS One. 2014 Jan 24; 9(1): e87265. doi: 10.1371/journal.pone.0087265.
60. Paul S. Phenotypic and functional plasticity of gamma-delta (γδ) T cells in inflammation and tolerance // S. Paul, A.K. Singh, Shilpi, G. Lal // Int. Rev. Immunol. 2014 Nov-Dec; 33(6): 537-58. doi: 10.3109/08830185.2013.863306.
61. Rahimpour A. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells –using MR1 tetramers / A. Rahimpour, H.F. Koay, A. Enders et al. // J. Exp. Med. 2015 Jun 29; 212(7): 1095-108. doi: 10.1084/jem.20142110.
62. Rampuria P., Lang M.L. CD1d-dependent expansion of NKT follicular helper cells in vivo and in vitro is a product of cellular proli–feration and differentiation // Int. Immunol. 2015 May; 27(5): 253-63. doi: 10.1093/intimm/dxv007.
63. Robinette M.L., Colonna M. Immune modules shared by innate lymphoid cells and T cells // J. Allergy Clin. Immunol. 2016 Nov; 138(5): 1243-1251. doi: 10.1016/j.jaci.2016.09.006.
64. Rossjohn J. Recognition of CD1d-restricted antigens by natural killer T cells / J. Rossjohn, D.G. Pellicci, O. Patel et al. // Nat. Rev. Immunol. 2012 Dec; 12(12): 845-57. doi: 10.1038/nri3328.
65. Salio M. Biology of CD1- and MR1-restricted T cells / M. Salio, J.D. Silk, E.Y. Jones, V. Cerundolo // Annu Rev. Immunol. 2014; 32: 323-66. doi: 10.1146/annurev-immunol-032713-120243.
66. Scanlon S.T. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation / S.T. Scanlon, S.Y. Thomas, C.M. Ferreira et al. // J. Exp. Med. 2011 Sep 26; 208(10): 2113-24. doi: 10.1084/jem.20110522.
67. Seyda M. T Cells Going Innate / M. Seyda, A. Elkhal, M. Quante et al. // Trends Immunol. 2016 Aug; 37(8): 546-56. doi: 10.1016/j.it.2016.06.004.
68. Silver J.S. Erratum: Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs / Silver J.S., Kearley J., Copenhaver A.M. et al. // Nat. Immunol. 2016 Jul 19; 17(8): 1005. doi: 10.1038/ni0816-1005c.
69. Silver J.S. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs / J.S. Silver, J. Kearley, A.M. Copenhaver et al. // Nat. Immunol. 2016 Jun; 17(6): 626-35. doi: 10.1038/ni.3443.
70. Small C.L. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung / C.L. Small, S. McCormick, N. Gill et al. // J. Immunol. 2008 Apr 15; 180(8): 5558-68. doi: 10.4049/jimmunol.180.8.5558.
71. Svedova J. TNF and CD28 Signaling Play Unique but Complementary Roles in the Systemic Recruitment of Innate Immune Cells after Staphylococcus aureus Enterotoxin A Inhalation / J. Svedova, N. Tsurutani, W. Liu et al. // J. Immunol. 2016 Jun 1; 196(11): 4510-21. doi: 10.4049/jimmunol.1600113.
72. Tebartz C. A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection / C. Tebartz, S.A. Horst, T. Sparwasser et al. // J. Immunol. 2015 Feb 1; 194(3): 1100-11. doi: 10.4049/jimmunol.1400196.
73. Turchinovich G., Hayday A.C. Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus interleukin-17-secreting γδ T cells // Immunity. 2011 Jul 22; 35(1): 59-68. doi: 10.1016/j.immuni.2011.04.018.
74. Two Types of Interleukin 17A-Producing γδ T Cells in Protection Against Pulmonary Infection With Klebsiella pneumoniae / Murakami T., Hatano S., Yamada H. et al. // J. Infect. Dis. 2016 Dec 1; 214(11): 1752-1761. doi: 10.1093/infdis/jiw443.
75. Uldrich A.P. CD1d-lipid antigen recognition by the γδ TCR / A.P. Uldrich, J. Le Nours, D.G. Pellicci et al. // Nat. Immunol. 2013 Nov; 14(11): 1137-45. doi: 10.1038/ni.2713.
76. Van Maele L. Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection / L. Van Maele, C. Carnoy, D. Cayet et al. // J. Infect. Dis. 2014 Aug 1; 210(3): 493-503. doi: 10.1093/infdis/jiu106.
77. Vavassori S. Butyrophilin 3A1 bings phosphorylated antigens and stimulates human γδ T cells // Vavassori S., Kumar A., Wan G.S. et al. // Nat. Immunol. 2013 Sep; 14(9): 908-16. doi: 10.1038/ni.2665.
78. Von Köckritz-Blickwede M. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model / M. von Köckritz-Blickwede, M. Rohde, S. Oehmcke et al. // Am. J. Pathol. 2008 Dec; 173(6): 1657-68. doi: 10.2353/ajpath.2008.080337.
79. Wands J.M. Distribution and leukocyte contacts of gamma–delta T cells in the lung // J.M. Wands, C.L. Roark, M.K. Aydintug et al. // J. Leukoc. Biol. 2005 Nov; 78(5): 1086-96. doi: 10.1189/jlb.0505244.
80. Wang H., Morita C.T. Sensor Function for Butyrophilin 3A1 in Prenyl Pyrophosphate Stimulation of human Vγ2Vδ2 T Cells / J. Immunol. 2015 Nov 15; 195(10): 4583-94. doi: 10.4049/jimmunol.1500314.
81. Wingender G., Sag D., Kronenberg M. NKT10 cells: a novel iNKT cell subset // Oncotarget. 2015 Sep 29; 6(29): 26552-3. doi: 10.18632/oncotarget.5270.
82. Wong E.B., Ndung’u T., Kasprowicz V.O. The role of mucosal-associated invariant T cells in infectious diseases // Immunology. 2017 Jan; 150(1): 45-54. doi: 10.1111/imm.12673.
83. Zhao H. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells / H. Zhao, W. Li, Y. Gao et al. // Toxico–logy. 2014 Nov 5; 325: 180-8. doi: 10.1016/j.tox.2014.09.006.
84. Ziegler C. The dynamics of T cells during persistent Staphylococcus aureus infection: from antigen-reactivity to in vivo anergy / C. Ziegler, O. Goldmann, E. Hobeika et al. // EMBO Mol. Med. 2011 Nov; 3(11): 652-66. doi: 10.1002/emmm.201100173.

/144-1.jpg)
/145-1.jpg)
/146-1.jpg)
/149-1.jpg)
/147-1.jpg)
/150-1.jpg)
/148-1.jpg)
/153-1.jpg)
/151-2.jpg)
/151-1.jpg)
/152-1.jpg)