Журнал "Гастроэнтерология" Том 54, №4, 2020
Вернуться к номеру
Age peculiarities of intestinal microbiocenosis disorders in the patients with ulcerative colitis and Crohn’s disease
Авторы: Yu.M. Stepanov, M.V. Titova, I.A. Klenina, O.M. Tatarchuk
SI “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”, Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
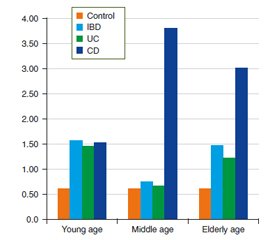
Актуальність. Хронічні запальні захворювання кишечника (ХЗЗК), а саме неспецифічний виразковий коліт (НВК) і хвороба Крона (ХК), залишаються однією з найбільш складних і актуальних проблем гастроентерології в усьому світі. На цей час залишається недостатньо вивченим питання про вплив мікрофлори кишечника та її змін на розвиток і прогресування запального процесу. Однак найбільш домінуюча етіологічна гіпотеза говорить про те, що ХЗЗК є результатом аномальної імунної відповіді на змінену мікробіоту кишечника під впливом факторів довкілля або патогенних мікроорганізмів у генетично схильного хазяїна. Зміни мікробіоти при ХЗЗК загальновизнані, але залежність цих змін від віку пацієнтів ще потребує дослідження. Мета дослідження: вивчити особливості дисбіозу кишечника й частоти синдрому надлишкового бактеріального росту (СНБР) у пацієнтів із ХЗЗК залежно від нозології та віку. Матеріали та методи. Обстежено 120 пацієнтів із ХЗЗК віком від 19 до 79 років, у середньому (43,90 ± 1,40) року; серед них 83 хворих на НВК, 37 — ХК. Усі хворі були розділені на групи залежно від нозології та віку. Хворим були проведені водневий дихальний тест для виявлення СНБР, бактеріологічне дослідження калу й хроматографія коротколанцюгових жирних кислот (КЖК) в копрофільтратах. Результати. Встановлені наявність глибоких змін якісного і кількісного складу мікрофлори товстої кишки і висока частота виявлення СНБР у пацієнтів із ХЗЗК. Виявлена залежність змін складу мікрофлори тонкої і товстої кишки у хворих від віку та нозології. Зниження концентрації біфідобактерій у вмісті товстого кишечника виявляли найчастіше в пацієнтів молодого віку з ХК, тоді як зниження кількості лактобактерій частіше визначалося у хворих похилого віку в обох нозологічних групах. Із віком зростала частота виявлення гемолітичних біоварів кишкової палички, умовно-патогенних ентеробактерій і грибів роду Candida. Спостерігалися зміни як сумарного загального змісту, так і показників окремих КЖК порівняно зі здоровими особами, що свідчило про пригнічення метаболічної активності нормальної мікрофлори. Зниження рівнів оцтової та масляної кислоти вказувало на виражене пригнічення продуцентів цих метаболітів. Висновки. Виявлено, що хворі похилого віку більш схильні до розвитку СНБР, зниження концентрації лактобактерій у вмісті товстої кишки, а також збільшення частоти виявлення умовно-патогенних ентеробактерій і грибів роду Candida. У хворих молодого віку переважно виявляється субкомпенсована форма дисбіозу зі зниженням концентрації біфідобактерій. Із віком також спостерігається пригнічення продуцентів оцтової і масляної кислоти. Результати цих досліджень дадуть можливість клініцистам більш ретельно підбирати терапевтичну тактику, а саме вплинуть на вибір препаратів, які модулюють мікробіоту кишечника, з урахуванням не тільки нозологічної форми, а й віку пацієнта.
Актуальность. Хронические воспалительные заболевания кишечника (ХВЗК), а именно неспецифический язвенный колит (НЯК) и болезнь Крона (БК), остаются одной из наиболее сложных и актуальных проблем гастроэнтерологии во всем мире. В настоящее время остается недостаточно изученным вопрос о влиянии микрофлоры кишечника и ее изменений на развитие и прогрессирование воспалительного процесса. Однако наиболее доминирующая этиологическая гипотеза говорит о том, что ХВЗК являются результатом аномального иммунного ответа на измененную микробиоту кишечника под воздействием факторов окружающей среды или патогенных микроорганизмов у генетически склонного хозяина. Изменения микробиоты при ХВЗК общепризнаны, но зависимость этих изменений от возраста пациентов еще нуждается в исследовании. Цель исследования: изучить особенности дисбиоза кишечника и частоты синдрома избыточного бактериального роста (СИБР) у пациентов с ХВЗК в зависимости от нозологии и возраста. Материалы и методы. Обследовано 120 пациентов с ХВЗК в возрасте от 19 до 79 лет, в среднем (43,90 ± 1,40) года; среди них 83 больных НЯК, 37 — БК. Все больные были разделены на группы в зависимости от нозологии и возраста. Больным были проведены водородный дыхательный тест для выявления СИБР, бактериологическое исследование кала и хроматография короткоцепочечных жирных кислот (КЖК) в копрофильтратах. Результаты. Установлены наличие глубоких изменений качественного и количественного состава микрофлоры толстой кишки и высокая частота выявления СИБР у пациентов с ХВЗК. Выявлена зависимость изменений состава микрофлоры тонкой и толстой кишки у больных от возраста и нозологии. Снижение концентрации бифидобактерий в содержимом толстого кишечника выявляли чаще всего у пациентов молодого возраста с БК, тогда как снижение количества лактобактерий чаще определялось у больных пожилого возраста в обеих нозологических группах. С возрастом росла частота выявления гемолитических биоваров кишечной палочки, условно-патогенных энтеробактерий и грибов рода Candida. Наблюдались изменения как суммарного общего содержания, так и показателей отдельных КЖК относительно здоровых лиц, что свидетельствовало об угнетении метаболической активности нормальной микрофлоры. Снижение уровней уксусной и масляной кислоты указывало на выраженное угнетение продуцентов этих метаболитов. Выводы. Выявлено, что больные пожилого возраста более склонны к развитию СИБР, снижению концентрации лактобактерий в содержимом толстой кишки, а также увеличению частоты выявления условно-патогенных энтеробактерий и грибов рода Candida. У больных молодого возраста преимущественно выявляется субкомпенсированная форма дисбиоза со снижением концентрации бифидобактерий. С возрастом также наблюдается угнетение продуцентов уксусной и масляной кислоты. Результаты данных исследований предоставят возможность клиницистам более тщательно подбирать терапевтическую тактику, а именно повлияют на выбор препаратов, которые модулируют микробиоту кишечника, с учетом не только нозологической формы, но и возраста пациента.
Background. Inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD) remain one of the most difficult and challenging gastroenterology problems. The impact of intestinal microflora and its changes on the development and progression of IBD has not been studied enough. The most dominant etiological hypothesis is that IBD is the result of an abnormal immune response to an altered intestinal microbiota caused by an environmental factor or pathogenic microorganisms in a genetically predisposed host. Altered gut microbiota dysbiosis in IBD is generally recognized, but the dependence of this change on the age still needs to be studied. The purpose of the study is to investigate the peculiarities of intestinal dysbiosis and the frequency of small intestinal bacterial overgrowth (SIBO) in patients with IBD depending on nosology and age. Materials and methods. One hundred and twenty patients with IBD aged 19 to 79 years (average (43.90 ± 1.40) years) were examined; among them 83 patients had UC, and 37 ones had CD. All patients were divided into two groups according to nosology and age. The patients underwent a hydrogen breath test to detect SIBO, bacteriological examination of feces, and short-chain fatty acid (SCFAs) chromatography in coprofiltrate. Results. The profound qualitative and quantitative changes of the colon microflora and high frequency of SIBO in patients with IBD were revealed. The dependence of changes in the microflora composition of the small and large intestine in a patient on age and nosology was discovered. The decrease in the concentration of Bifidobacteria in the content of the colon was found mostly in young patients with CD, while the decrease in the number of Lactobacilli was mostly found in elderly patients in both groups. The frequency of hemolytic biovars of Escherichia coli, opportunistic enterobacteria, and fungi of the genus Candida in the colon increased with age. There were changes in both the total content and indices of some SCFAs in patients of both groups versus healthy persons that indicated the suppression of the metabolic activity of normal microflora in patients. Decreased levels of acetic and butyric acid indicated the severity of suppression of the production of these metabolites. Conclusions. The elderly patients were found to have a greater tendency to develop SIBO, a decrease in the colon Lactobacilli concentration, as well as an increased frequency of detection of conditionally pathogenic flora and fungi of the genus Candida. In young patients, there is mainly a subcompensated form of dysbiosis with a decrease in the concentration of Bifidobacteria. With age, there is also suppression of acetic and butyric acid production. The results of this study will allow clinicians to select therapeutic tactics in these patients more carefully, namely, will influence the choice of drugs that modulate the intestinal microbiota, taking into account not only the nosology but also the patient’s age.
хронічні запальні захворювання кишечника; мікрофлора кишечника; синдром надлишкового бактеріального росту; коротколанцюгові жирні кислоти; неспецифічний виразковий коліт; хвороба Крона
хронические воспалительные заболевания кишечника; микрофлора кишечника; синдром избыточного бактериального роста; короткоцепочечные жирные кислоты; неспецифический язвенный колит; болезнь Крона
inflammatory bowel disease; intestinal microflora; small intestinal bacterial overgrowth; short-chain fatty acids; ulcerative colitis; Crohn’s disease
Introduction
Materials and methods
Results
/20_2.jpg)
/22_2.jpg)
Conclusions
- Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015. 12 (12). 720-727. doi: 10.1038/nrgastro.2015.150.
- Nishida A., Inoue R., Inatomi O., Bamba S., Naito Y., Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018. 11 (1). 1-10. doi: 10.1007/s12328-017-0813-5.
- Khan I., Ullah N., Zha L. et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019. 8 (3). 126. Published 2019 Aug 13. doi: 10.3390/pathogens8030126.
- Sartor R.B., Wu G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017. 152 (2). 327-339. e4. doi: 10.1053/j.gastro.2016.10.012.
- Takahashi K., Nishida A., Fujimoto T. et al. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease [published correction appears in Digestion. 2016. 93 (2). 174]. Digestion. 2016. 93 (1). 59-65. doi: 10.1159/000441768.
- Bourkovskaya V.A., Beloborodova E.I., Akimova L.A. et al. Narushenie mikrobiocenoza kishechnika pri hronicheskih vospalitel’nyh zabolevanijah kishechnika i absorbcionnaja funkcija tonkoj kishki [Disturbed intestinal microcenosis in chronic inflammatory intestinal diseases and absorption function of the small intestine]. Sibirskij medicinskij zhurnal. 2009. 24 (4–2). 40-45. (In Russian).
- Vemuri R., Gundamaraju R., Shastri M.D. et al. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. Biomed. Res. Int. 2018. 2018. 4178607. Published 2018 Feb 26. doi: 10.1155/2018/4178607.
- Bel’mer S.V., Ardatskaja M.D., Akopjan A.N. Korotkocepochechnye zhirnye kisloty v lechenii funkcional’nyh zabolevanij kishechnika u detej. Teoreticheskoe obosnovanie i prakticheskoe primenenie. M.: Prima Print, 2015. 48. (In Russian).
- Stepanov Yu.M., Titova M.V., Tatarchuk O.M. Porushennja mіkrobіocenozu tovstogo kishechnika і chastota sindromu nadlishkovogo bakterіal’nogo rostu u hvorih na hronіchnі zapal’nі zahvorjuvannja kishechnika [Large intestine microbiocoenosis disorders and the incidence of small intestinal bacterial overgrowth syndrome in patients suffering from inflammatory bowel diseases]. Gastroenterologia. 2020. № 1 (54). 44-50. (In Ukrainian). doi: 10.22141/2308-2097.54.1.2020.199141.
- O’Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015. 350 (6265). 1214-1215. doi: 10.1126/science.aac8469.
- Green N., Miller T., Suskind D., Lee D. A Review of Dietary Therapy for IBD and a Vision for the Future. Nutrients. 2019. 11 (5). 947. Published 2019 Apr 26. doi: 10.3390/nu11050947.
- Tarasiuk A., Eibl G. Nutritional support and probiotics as potential treatment in IBD [published online ahead of print, 2020 May 3]. Curr. Drug. Targets. 2020. doi: 10.2174/1389450121666200504075519.
- Rezaie A., Buresi M., Lembo A. et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017. 112 (5). 775-784. doi: 10.1038/ajg.2017.46.

/20.jpg)
/21.jpg)
/22.jpg)