Журнал "Гастроэнтерология" Том 58, №1, 2024
Вернуться к номеру
Оцінка функціонального стану мікробіоти кишечника в пацієнтів із метаболічно-асоційованою жировою хворобою печінки в поєднанні з цукровим діабетом 2 типу
Авторы: O.K. Didyk
Bogomolets National Medical University, Kyiv, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
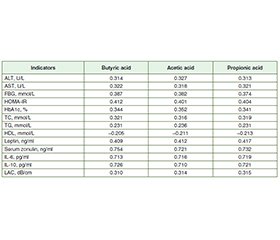
Актуальність. Метою дослідження було оцінити функціональний стан мікробіоти кишечника в осiб із метаболічно-асоційованою жировою хворобою печінки (МАЖХП) у поєднанні з цукровим діабетом (ЦД) 2 типу. Матеріали та методи. У дослідженні взяв участь 71 пацієнт із МАЖХП, поєднаною з ЦД 2 типу, яких було обстежено та розділено на 2 групи. До першої групи ввійшли 39 осіб із МАЖХП у поєднанні з ЦД 2 типу без синдрому надмірного бактеріального росту (СНБР), до другої — 32 пацієнти з МАЖХП, поєднаною з ЦД 2 типу та СНБР. Групу контролю становили 25 практично здорових осіб. Уміст зонуліну в сироватці крові визначали методом імуноферментного аналізу (ІФА) з використанням тест-систем IDK Zonulin ELISA, KR5601. Концентрацію інтерлейкіну (ІЛ) 6 та ІЛ-10 у сироватці крові оцінювали за допомогою ІФА та тест-систем Human Interleukin 6 and 10 ELISA Kit. Рівень коротколанцюгових жирних кислот у калі визначали методом газової хроматографії з мас-спектрометрією на газовому хроматографі PerkinElmer Clarus 680 GC. Результати. У хворих першої та другої груп установлено підвищення печінкових амінотрансфераз, загального холестерину, тригліцеридів, глюкози крові натще, інсуліну, індексу HOMA-IR, глікозильованого гемоглобіну, зонуліну сироватки крові, лептину, ІЛ-6 і коефіцієнта затухання ультразвуку та зниження рівня ліпопротеїнів високої щільності. За результатами стеатометрії зафіксовано тяжкий ступінь стеатозу (S3) у пацієнтів із МАЖХП у поєднанні з ЦД 2 типу й СНБР та без СНБР. При кількісному дослідженні рівня масляної кислоти у фекаліях встановлено його вірогідне зменшення в 2,3 раза в першій групі та в 3,4 раза в другій порівняно з групою контролю (p < 0,001). У хворих другої групи вміст масляної кислоти був знижений в 1,4 раза порівняно з пацієнтами першої групи (p < 0,001). При порівнянні рівня оцтової кислоти виявлено його вірогідне підвищення в 1,2 раза в першій групі та в 1,3 раза в другій порівняно з контрольною групою (p < 0,001). Установлено збільшення концентрації пропіонової кислоти в 1,3 раза в першій групі та в 1,5 раза в другій порівняно з групою контролю (p < 0,05). У хворих першої та другої груп спостерігалося вірогідне підвищення рівня оцтової кислоти в 1,3 раза відносно контрольної групи (p < 0,001). При порівнянні концентрацій пропіонової та оцтової кислоти в другій групі спостерігалося їх збільшення в 1,1 раза порівняно з першою (p < 0,05). Аналіз коефіцієнтів кореляції в осіб із МАЖХП у поєднанні з ЦД 2 типу й СНБР та без СНБР виявив прямий пропорційний сильний зв’язок між рівнями масляної, оцтової та пропіонової кислот і сироваткового зонуліну, ІЛ-6, ІЛ-10. При вивченні кореляцій установлено прямий помірний зв’язок між умістом коротколанцюгових жирних кислот і лептину, показниками ліпідного (загальний холестерин, тригліцериди), вуглеводного обміну (глюкоза крові, HOMA-IR, HbA1c) і зворотну слабку кореляцію з рівнем ліпопротеїнів високої щільності. Зафіксовано прямий помірний кореляційний зв’язок між рівнями коротколанцюгових жирних кислот та показниками функціональної активності печінки (аланінамінотрансфераза, аспартатамінотрансфераза), ступенем стеатозу в осіб із МАЖХП у поєднанні з ЦД 2 типу й СНБР та без СНБР. Висновки. У пацієнтів із МАЖХП, поєднаною з ЦД 2 типу й СНБР та без СНБР, виявили підвищену кишкову проникність та гіперлептинемію. При дослідженні функціонального стану мікробіоти кишечника встановлено підвищення рівнів пропіонової та оцтової кислот та зниження масляної, що свідчить про збільшення кількості Bacteroidetes у кишечнику та зменшення Firmicutes. У пацієнтів із МАЖХП у поєднанні з ЦД 2 типу й СНБР та без СНБР виявлено прямий пропорційний сильний кореляційний зв’язок між рівнями масляної, оцтової та пропіонової кислот, зонуліну в сироватці крові та IЛ-6, IЛ-10, що асоціюється з прозапальними і протизапальними процесами в слизовій оболонці кишечника й порушенням проникності кишкового бар’єра. Установлено, що концентрації коротколанцюгових жирних кислот корелюють з показниками функціональної активності печінки (аланінамінотрансфераза, аспартатамінотрансфераза), рівнем лептину, показниками вуглеводного та ліпідного обміну, ступенем стеатозу печінки.
Background. The purpose of the study was to assess the functional state of gut microbiota in patients with metabolic-associated fatty liver disease (MAFLD) combined with type 2 diabetes mellitus (T2DM). Materials and methods. The prospective interventional randomized study included 71 patients with MAFLD in combination with T2DM, who were examined and divided into the 2 groups. The first group included 39 people with MAFLD and T2DM without small intestinal bacterial overgrowth (SIBO). The second group consisted of 32 patients with MAFLD in combination with T2DM and SIBO. The control group included 25 practically healthy patients. The content of serum zonulin was determined by enzyme-linked immunosorbent assay (ELISA) using test systems IDK Zonulin ELISA, KR5601. Serum concentration of interleukin (IL) 6 and IL-10 was assessed by the ELISA method using the Human Interleukin 6 and 10 ELISA Kit test systems. The content of short-chain fatty acids in feces was determined by gas chromatography with mass spectrometry in the PerkinElmer Clarus 680 GC Gas Chromatograph. Results. Patients of the first and second groups had an increase in hepatic aminotransferases, total cholesterol, triglycerides, fasting blood glucose, insulin, HOMA-IR, glycosylated hemoglobin, serum zonulin, leptin, IL-6 and liver attenuation coefficient and a decrease in high-density lipoprotein. The results of steatometry revealed a severe degree of hepatic steatosis (S3) in patients with MAFLD combined with T2DM and SIBO and without SIBO. During the quantitative study of the level of butyric acid in feces, it was found to be significantly reduced, by 2.3 times in the first group and by 3.4 times in the second one, compared to the controls (p < 0.001). The content of butyric acid was decreased by 1.4 times in the second group compared to the first one (p < 0.001). When evaluating the level of acetic acid, it was found to be significantly increased compared to the controls, by 1.2 times in the first group and by 1.3 times in the second one (p < 0.001). The concentration of propionic acid was increased by 1.3 times in the first group and by 1.5 times in the second one compared the control group (p < 0.05). When comparing the level of acetic acid, a significant increase by 1.3 times was observed in patients of the first and second groups compared to patients of the control group (p < 0.001). The content of propionic and acetic acids was increased by 1.1 times in the second group compared to patients of the first group (p < 0.05). When analyzing the correlation coefficients in patients with MAFLD combined with T2DM and SIBO and without SIBO, a direct proportional strong correlation was revealed between the levels of butyric, acetic and propionic acids and serum zonulin, IL-6, IL-10. A direct moderate correlation was found between the content of short-chain fatty acids and leptin, indicators of lipid metabolism (total cholesterol, triglycerides), carbohydrate metabolism (fasting blood glucose, HOMA-IR, HbA1c) and inverse weak correlation with high-density lipoprotein. A direct moderate correlation was revealed between levels of short-chain fatty acids and indicators of functional activity of the liver (alanine aminotransferase, aspartate aminotransferase), liver attenuation coefficient in patients with MAFLD combined with T2DM and SIBO and without SIBO. Conclusions. Increased intestinal permeability and hyperleptinemia have been found in patients with МAFLD combined with T2DM and SIBO and without SIBO. When studying the functional state of the gut microbiota, an increase was found in propionic and acetic acids and a decrease in butyric acid, which indicates an increase in the number of Bacteroidetes in the intestine and a decrease in Firmicutes. Patients with MAFLD combined with Т2DM and SIBO and without SIBO had a direct proportional strong correlation between the levels of butyric, acetic and propionic acids and serum zonulin, IL-6, IL-10, which is associated with pro-inflammatory and anti-inflammatory processes in the intestinal mucosa and a violation of the permeability of the intestinal barrier. It was found that concentrations of short-chain fatty acids correlate with indicators of functional activity of the liver (alanine aminotransferase, aspartate aminotransferase), leptin, indicators of carbohydrate and lipid metabolism, degree of hepatic steatosis.
кишкова проникність; цукровий діабет 2 типу; лептин; коротколанцюгові жирні кислоти; зонулін; метаболічно-асоційована жирова хвороба печінки
intestinal permeability; type 2 diabetes mellitus; leptin; short-chain fatty acids; zonulin; metabolic-associated fatty liver disease
Introduction
Materials and methods
Results
/52.jpg)
Discussion
Conclusions
- Leung C. et al. The role of the gut microbiota in NAFLD. Nature Reviews. Gastroenterology & Hepatology. 2016. Vol. 13. No. 7. P. 412-425. doi: 10.1038/nrgastro.2016.85.
- Augustyn M. et al. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. Clinical and Experimental Hepatology. 2019. Vol. 5. No. 1. P. 1-10. doi: 10.5114/ceh.2019.83151.
- Kessoku T. et al. Endotoxins and Non-Alcoholic Fatty Liver Disease. Frontiers in Endocrinology. 2021. Vol. 12. P. 770-786. doi: 10.3389/fendo.2021.770986.
- Ferolla S.M. et al. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients. 2014. Vol. 6. No. 12. P. 5583-99. doi: 10.3390/nu6125583.
- Silva Y.P. et al. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Frontiers in Endocrinology. 2020. Vol. 11. No. 25. doi: 10.3389/fendo.2020.00025.
- Wolever T.M., Brighenti F., Royall D., Jenkins A.L., Jenkins D.J. Effect of rectal infusion of short chain fatty acids in human subjects. Am J Gastroenterol. 1989. Vol. 84. No. 9. Р. 1027-33.
- Tolhurst G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012. Vol. 61. No. 2. P. 364-71. doi: 10.2337/db11-1019.
- ElSayed N.A., Aleppo G., Aroda V.R. et al. Erratum. 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023;46(Suppl. 1):S19-S40. Diabetes Care. 2023. Vol. 46. No. 5. P. 1106. doi: 10.2337/dc23-er05.
- Vallianou N. et al. Understanding the Role of the Gut Micro–biome and Microbial Metabolites in Non-Alcoholic Fatty Liver Di–sease: Current Evidence and Perspectives. Biomolecules. 2021. Vol. 12. No. 1. P. 56. doi: 10.3390/biom12010056.
- Gao Zh. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009. Vol. 58. No. 7. P. 1509-17. doi: 10.2337/db08-1637.
- Vinolo M.A.R. et al. Regulation of inflammation by short chain fatty acids. Nutrients. 2011. Vol. 3. No. 10. P. 858-76. doi: 10.3390/nu3100858.
- Den Besten G. et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes. 2015. Vol. 64. No. 7. P. 2398-408. doi: 10.2337/db14-1213.
- Miele L. et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2009. Vol. 49. No. 6. P. 1877-87. doi: 10.1002/hep.22848.
- Minokoshi Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002. Vol. 415. No. 6869. P. 339-43. doi: 10.1038/415339a.

/51.jpg)
/51_2.jpg)
/52_2.jpg)