Хемокины
Хемокины семейства CC
CCR1, CCL3
Экспрессия CCL3 хемокина семейства CC эпителиальными клетками во время стафилококковой инфекции за счет его взаимодействия с клеточным рецептором CCR1 способствует рекрутированию Th1-клеток и, вероятно, альвеолярных макрофагов в очаг поражения [11, 58].
CCR2, CCL2
Альвеолоциты при инфицировании бактериями Staphylococcus aureus продуцируют CCL2 [4], основной функцией которого является высвобождение моноцитов из костного мозга и транслокация их в кровеносное русло. Практически двукратное увеличение представительства моноцитов и макрофагов в бронхоальвеолярной жидкости отмечается уже через 4 часа после инфицирования Staphylococcus aureus [15]. Известно, что CCL2 также продуцируется макрофагами, лимфоцитами, базофилами, эпителиальными клетками, эндотелиальными клетками и фибробластами [24, 45].
Хемокины семейства CXC
CXCR1 и CXCR2, CXCL1 и CXCL2
Показано, что развитие стафилококковой инфекции сопровождается повышением продукции хемокинов CXCL1 и CXCL2, участвующих в рекрутинге нейтрофилов [59]. Необходимо отметить, что возбуждение Tlr2 (у мышей) Pam3CSK4 MRSA может ингибировать экспрессию хемокинов CXCL1 и CXCL2 и снижать активность рекрутирования нейтрофилов в очаг поражения легкого [9].
IL-8/СХСL8
Влияние факторов вирулентности Staphylococcus aureus, в частности поверхностного протеина A, лейкоцидина Пантона — Валентина, сопровождается высвобождением IL-1 и IL-8/СХСL8 в ткани легкого. Данные цитокины являются основными хемоаттрактантами нейтрофилов [33]. Увеличение количества нейтрофилов в костном мозге (незрелые формы) и периферической крови на 63 и 81 % соответственно происходит в более поздний период заболевания — через 16 часов после инфицирования Staphylococcus aureus [15]. Во время стафилококковой инфекции IL-8/СХСL8 первично продуцируется эпителиоцитами слизистой оболочки респираторного тракта [1]. В последующем основными продуцентами IL-8/СХСL8 становятся нейтрофилы. Бактерии Staphylococcus aureus могут ингибировать продукцию IL-8/СХСL8, способствуя собственному выживанию [64].
Роль хемокинов при стафилококковой пневмонии представлена на рис. 1.
Антимикробные пептиды
В респираторном тракте бактерицидное действие оказывают многочисленные антимикробные пептиды и протеины, которые отличаются особенностями механизмов индукции их синтеза и действия (табл. 1) [36].
Наиболее функционально значимыми АМП, осуществляющими бактерицидную функцию в респираторном тракте человека, являются лизоцим и дефензины [19, 48], однако бактерии Staphylococcus aureus обладают высокой резистентностью к их бактерицидной активности [2, 26, 30, 31]. В отличие от дефензинов LL-37 обладает выраженной бактерицидной антистафилококковой активностью и синергизмом действия с дефензинами, лактоферрином и лизоцимом [8, 16, 37].
LL-37
Общая характеристика
Человеческий катионный антимикробный пептид 18 кДа (hCAP18), который кодируется геном CAMP, расположенным на хромосоме 3 (3p21.3), и его N-терминальный активный фрагмент, состоящий из 37 аминокислотных остатков и представляющий собой зрелый пептид LL-37, впервые были обнаружены во вторичных нейтрофильных гранулах [13]. Экспрессия CAMP носит выраженный витамин-D-зависимый характер [46].
Пептид LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) является единственным известным представителем кателицидинового семейства антимикробных пептидов в организме человека [57]. Его молекула представляет амфифильную α-спиральную третичную структуру и существует в виде мономерных и олигомерных форм, которые находятся в количественном равновесии. Благодаря катионности (+6) и гидрофобности своей молекулы пептид LL-37 взаимодействует с несущей отрицательный заряд стенкой бактерий и в последующем формирует поры. Бактерии погибают в результате истечения содержимого через сформированные поры. Также пептид LL-37 обладает способностью непосредственно взаимодействовать с LPS бактерий [21, 43]. Продуцентами hCAP18/LL-37 в респираторном тракте являются эпителиальные клетки, альвеолярные макрофаги, нейтрофилы, моноциты и макрофаги. Активация TLR2, TLR4 и TLR9 приводит к продукции LL-37. Считают, что основную антибактериальную роль пептид LL-37 играет в раннем периоде инфицирования и практически малоэффективен в поздний период инфекционного процесса [28, 40, 44].
Бактерицидная и антибиопленочная активность
Пептид LL-37 демонстрирует достаточно выраженную бактерицидную активность по отношению как к золотистому стафилококку, так и к другим патогенным бактериям. Пептид LL-37 под действием сериновых протеаз расщепляется на более мелкие фрагменты, такие как KR-20, КС-30, РК-31, LL-23, КС-27, МР-29 и КС-22, которые также обладают антибактериальной активностью (табл. 2) [57].
Пептид LL-37 в бактерицидных концентрациях присутствует в инфицированных регионах респираторного тракта. Увеличение концентрации LL-37 во время стафилококковой инфекции предшествует повышению уровня α-дефензинов в бронхоальвеолярной жидкости [6].
Также пептид LL-37 и его фрагменты препятствуют формированию биопленок патологическими бактериями (табл. 3).
Пептид LL-37 in vitro ингибирует образование биопленки золотистым стафилококком при значительно более низких концентрациях, чем требуется для ингибирования роста колонии или для индукции гибели бактерий [14, 41].
Также LL-37 обладает противогрибковым, противовирусным действием.
Влияние на клетки макроорганизма
Пептид LL-37 оказывает влияние на эпителиоциты, иммуноциты, усиливая процесс элиминации патогена из макроорганизма [17].
Продемонстрировано, что пептид LL-37 реализует свое действие, взаимодействуя с G-протеинсвязанными рецепторами (G protein-coupled receptor — GPCR); рецепторами тирозиновых киназ; трансмембранными каналами, TLR [54]. Пептид LL-37 непосредственно усиливает активность экспрессии 29 генов и подавляет транскрипцию 20 генов. Среди активируемых генов высокой чувствительностью к влиянию LL-37 обладают гены, кодирующие хемокины и рецепторы хемокинов. Пептид LL-37 усиливает экспрессию моноцитарного хемоаттрактантного протеина 1 (monocyte chemoattractant protein 1 — MCP-1/CCL2) и TNF-α моноцитами, IL-8/CXCL8 — эпителиальными клетками респираторного тракта человека. Также –LL-37 способствует повышению уровня экспрессии –CXCR-4, CCR2, IL-8RB (табл. 4) и подавляет экспрессию ДНК-репарирующих протеинов и субъединиц потенциалзависимых натриевых каналов (табл. 5) [47].
Эффекты взаимодействия LL-37 с рецепторами GPCR
Продемонстрировано, что пептид LL-37 взаимодействует с GPCR: формилпептидным рецептором 2 (formyl peptide receptor 2 — FPR2), хемокиновым рецептором CXCR2, MrgX2 (MAS related GPR family member X2), пуринергическим рецептором P2Y11 (purinergic receptor P2Y11).
Взаимодействие пептида LL-37 с FPR2 эпителиальных клеток способствует повышению барьерной функции эпителия респираторного тракта [54], инициирует хемотаксис нейтрофилов, моноцитов и Т-лимфоцитов [55]. Пептид LL-37, активируя FPR2, подавляет апоптоз нейтрофилов, вызывает продукцию лейкотриенов B4 (LTB4), генерацию АКМ, индуцирует формирование нейтрофильных внеклеточных ловушек [39]. Возбуждение LL-37 рецептора FPR2 эозинофилов вызывает высвобождение цистеиновых лейкотриенов [49].
Пептид LL-37, взаимодействуя с хемокиновым рецептором CXCR2, который представляет собой GPCR, чувствительный к токсину коклюша, способствует рекрутированию нейтрофилов [63].
Как β-дефензины человека и вещество Р, пептид LL-37 может выступать в качестве агониста рецептора MrgX2 тучных клеток [60]. Пептид LL-37 активирует тучные клетки и индуцирует высвобождение гистамина [22].
Эффекты взаимодействия LL-37 с рецепторами тирозиновых киназ
Пептид LL-37 способствует возбуждению таких рецепторов тирозиновых киназ, как рецептор эпидермального фактора роста (epidermal growth factor receptor — EGFR) и рецептор инсулиноподобного фактора I (insulin-like growth factor 1 receptor — IGF1R) [20], за счет активации ADAM-9, ADAM-10, ADAM-12, ADAM-15, ADAM-17 и ADAM-19 [54]. Активация ADAM способствует высвобождению мембраносвязанных EGF, TGF-α, которые в дальнейшем, связываясь с EGFR, приводят к продукции MUC5AC муцина [62]. Трансактивация –LL-37 рецептора EGFR индуцирует миграцию эпителио–цитов и, таким образом, способствует репарации эпителия, а TGF-α повышает уровень активности воспаления [21]. LL-37-индуцированное возбуждение IGF1R способствует инвазии злокачественных клеток [20].
Эффекты взаимодействия LL-37 с трансмембранными каналами
Пептид LL-37 также взаимодействует с человеческим пуринергическим рецептором Р2Х7R, который относится к семейству ионотропных АТФ-зависимых рецепторов и высоко экспрессируется иммуноцитами. Активация Р2Х7R приводит к открытию канала для таких катионов, как кальций, натрий и калий [32]. Возбуждение P2X7R, в том числе и LL-37-индуцированное, сопровождается моноцитарной продукцией IL-1β, IL-2, IL-6, IL-18, TNF-α, CXCL3, образованием активных азотсодержащих метаболитов (ААМ) [3, 5, 27, 35], IL-8/CXCL8 [38], PGE2 [10]. Также LL-37 способствует продукции LTB4 и TXA2 макрофагами через P2X7R [56]. Под влиянием LL-37 усиливается продукция CCL2, рекрутирующего моноциты и Т-клетки [18].
Кроме того, LL-37 предопределяет дифференцировку моноцитов человека в фенотип M1 и способствует продукции ими IL-12p40 [53].
Взаимодействие LL-37 с Р2Х7R макрофагов приводит к интернализации комплекса LL-37/Р2Х7R, что способствует клиренсу внутриклеточно расположенных бактерий [50].
Эффекты взаимодействия LL-37 с рецепторами TLR
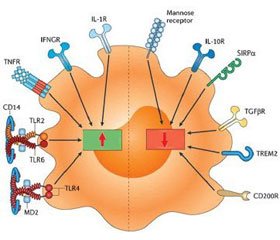
Пептид LL-37 изменяет активность возбуждения бактериальными лигандами TLR, модулируя выраженность воспалительного процесса (табл. 6).
Действие пептида LL-37 при стафилококковой пневмонии схематически представлено на рис. 2.
Sae-Hae Kim и соавт. [29] предполагают, что –LL-37, с учетом его непосредственной антибактериальной активности и способности модулировать продукцию провоспалительных цитокинов, является перспективным кандидатом, который может быть положен в основу создания лекарственного средства для лечения септических состояний.
Конфликт интересов. Авторы заявляют об отсутствии какого-либо конфликта интересов при подготовке данной статьи.
Список литературы
1. Al Alam D. Impaired interleukin-8 chemokine secretion by staphylococcus aureus-activated epithelium and T-cell chemotaxis in cystic fibrosis / D. Al Alam, G. Deslee, C. Tournois et al. // Am. J. Respir. Cell. Mol. Biol. 2010 Jun; 42(6): 644-50. doi: 10.1165/rcmb.2008-0021OC.
2. Andersson D.I. Mechanisms and consequences of bacterial resistance to antimicrobial peptides / D.I. Andersson, D. Hughes, J.Z. Kubicek-Sutherland et al. // Drug. Resist. Updat. 2016 May; 26: 43-57. doi: 10.1016/j.drup.2016.04.002.
3. Arulkumaran N., Unwin R.J., Tam F.W.A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases // Expert Opin. Investig. Drugs. 2011 Jul; 20(7): 897-915. doi: 10.1517 /13543784.2011.578068.
4. Athale J. Nrf2 promotes alveolar mitochondrial bioge–nesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice / J. Athale, A. Ulrich, N.C. MacGarvey et al. // Free Radic. Biol. Med. 2012 Oct 15; 53(8): 1584-94. doi: 10.1016/j.freeradbiomed.2012.08.009.
5. Baudelet D. Involvement of the P2X7 purinergic receptor in inflammation: an update of antagonists series since 2009 and their promi–sing therapeutic potential / D. Baudelet, E. Lipka, R. Millet, A. Ghinet // Curr. Med. Chem. 2015; 22(6): 713-29. doi: 10.2174/0929867322666141212120926.
6. Braff M.H. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis / M.H. Braff, A.L. Jones, S.J. Skerrett, C.E. Rubens // J. Infect. Dis. 2007 May 1; 195(9): 1365-72. doi: 10.1086/513277.
7. Chen Q.X. Silencing airway epithelial cell-derived hepcidin exa–cerbates sepsis induced acute lung injury / Q.X. Chen, S.W. Song, Q.H. Chen et al. // Crit. Care. 2014 Aug 6; 18(4): 470. doi: 10.1186/s13054-014-0470-8.
8. Chen X. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli / X. Chen, F. Niyonsaba, H. Ushio et al. // J. Dermatol. Sci. 2005 Nov; 40(2): 123-32. doi: 10.1016/j.jdermsci.2005.03.014.
9. Chen Y.G. Control of Methicillin-Resistant Staphylococcus aureus Pneumonia Utilizing TLR2 Agonist Pam3CSK4 / Y.G. Chen, Y. Zhang, L.Q. Deng et al. // PLoS One. 2016 Mar 14; 11(3): e0149233. doi: 10.1371/journal.pone.0149233.
10. Chotjumlong P. Involvement of the P2X7 purinergic receptor and c-Jun N-terminal and extracellular signal-regulated kinases in cyclooxygenase-2 and prostaglandin E2 induction by LL-37 / P. Chotjumlong, J.G. Bolscher, K. Nazmi et al. // J. Innate Immun. 2013; 5(1): 72-83. doi: 10.1159/000342928.
11. Cohen T.S. Staphylococcus aureus α toxin potentiates opportunistic bacterial lung infections / T.S. Cohen, J.J. Hilliard, O. Jones-Nelson et al. // Sci Transl. Med. 2016 Mar 9; 8(329): 329ra31. doi: 10.1126/scitranslmed.aad9922.
12. Coorens M. Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis / M. Coorens, M.R. Scheenstra, E.J. Veldhuizen, H.P. Haagsman // Sci Rep. 2017 Jan 19; 7: 40874. doi: 10.1038/srep40874.
13. Cowland J.B., Johnsen A.H., Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules // FEBS Lett. 1995 Jul 10; 368(1): 173-6. doi: 10.1016/0014-5793(95)00634-L.
14. Dean S.N., Bishop B.M., van Hoek M.L. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm acti–vity against Staphylococcus aureus // BMC Microbiol. 2011 May 23; 11: 114. doi: 10.1186/1471-2180-11-114.
15. Desouza I.A. Inflammatory mechanisms underlying the rat pulmonary neutrophil influx induced by airway exposure to staphylococcal enterotoxin type A / I.A. Desouza, C.F. Franco-Penteado, E.A. Camargo et al. // Br. J. Pharmacol. 2005 Nov; 146(6): 781-91. doi: 10.1038/sj.bjp.0706393.
16. Dorschner R.A. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides / R.A. Dorschner, B. Lopez-Garcia, A. Peschel et al. // FASEB J. 2006 Jan; 20(1): 35-42. doi: 10.1096/fj.05-4406com.
17. Fabisiak A., Murawska N., Fichna J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity // Pharmacol Rep. 2016 Aug; 68(4): 802-8. doi: 10.1016/j.pharep.2016.03.015.
18. Flick-Smith H.C. Assessment of antimicrobial peptide LL-37 as a post-exposure therapy to protect against respiratory tularemia in mice / Flick-Smith H.C., Fox M.A., Hamblin K.A. et al. // Peptides. 2013 May; 43: 96-101. doi: 10.1016/j.peptides.2013.02.024.
19. Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract // J. Clin. Invest. 2002 Mar; 109(6): 693-7. doi: 10.1172/JCI15218.
20. Girnita A. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor / A. Girnita, H. Zheng, A. Grönberg, L. Girnita, M. Ståhle // Oncogene. 2012 Jan 19; 31(3): 352-65. doi: 10.1038/onc.2011.239.
21. Golec M. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide // Ann. Agric. Environ. Med. 2007; 14(1): 1-4. PMID: 17655171.
22. Gupta K., Subramanian H., Ali H. Modulation of host defense peptide-mediated human mast cell activation by LPS // Innate Immun. 2016 Jan; 22(1): 21-30. doi: 10.1177/1753425915610643.
23. Gutsmann T. Interaction between antimicrobial peptides and mycobacteria // Biochim. Biophys. Acta. 2016 May; 1858(5): 1034-43. doi: 10.1016/j.bbamem.2016.01.031.
24. Izykowski N. Organizing pneumonia in mice and men / N. Izykowski, M. Kuehnel, K. Hussein et al. // J. Transl. Med. 2016 Jun 10; 14(1): 169. doi: 10.1186/s12967-016-0933-6.
25. Jacobsen A.S., Jenssen H. Human cathelicidin LL-37 prevents bacterial biofilm formation // Future Med. Chem. 2012 Aug; 4(12): 1587-99. doi: 10.4155/fmc.12.97.
26. Joo H.S., Fu C.I., Otto M. Bacterial strategies of resistance to antimicrobial peptides // Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016 May 26; 371(1695). pii: 20150292. doi: 10.1098/rstb.2015.0292.
27. Kahlenberg J.M., Kaplan M.J. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease // J. Immunol. 2013 Nov 15; 191(10): 4895-901. doi: 10.4049/jimmunol.1302005.
28. Karadottir H. Cyclic mechanical stretch down-regulates cathelicidin antimicrobial peptide expression and activates a pro-inflammatory response in human bronchial epithelial cells / H. Karadottir, N.N. Kulkarni, T. Gudjonsson et al. // Peer J. 2015 Dec 7; 3: e1483. doi: 10.7717/peerj.1483.
29. Kim S.H., Lee H.Y., Jang Y.S. Expression of the ATP-gated P2X7 Receptor on M Cells and Its Modulating Role in the Mucosal Immune Environment // Immune Netw. 2015 Feb; 15(1): 44-9. doi: 10.4110/in.2015.15.1.44.
30. Kraus D., Peschel A.Staphylococcus aureus evasion of innate antimicrobial defense // Future Microbiol. 2008 Aug; 3(4): 437-51. doi: 10.2217/17460913.3.4.437.
31. Kubicek-Sutherland J.Z. Antimicrobial peptide exposure selects for Staphylococcus aureus resistance to human defence peptides / J.Z. Kubicek-Sutherland, H. Lofton, M. Vestergaard et al. // J. Antimicrob. Chemother. 2017 Jan; 72(1): 115-127. doi: 10.1093/jac/dkw381.
32. Kumagai S. Cathelicidin antimicrobial peptide inhibits fibroblast migration via P2X7 receptor signaling / S. Kumagai, K. Matsui, H. Kawaguchi et al. // Biochem. Biophys. Res. Commun. 2013 Aug 9; 437(4): 609-14. doi: 10.1016/j.bbrc.2013.07.010.
33. Labrousse D. Kineret®/IL-1ra blocks the IL-1/IL-8 inflammatory cascade during recombinant Panton Valentine Leukocidin-triggered pneumonia but not during S. aureus infection / D. Labrousse, M. Perret, D. Hayez et al. // PLoS One. 2014 Jun 6; 9(6): e97546. doi: 10.1371/journal.pone.0097546.
34. Lai H.C. The assessment of host and bacterial proteins in sputum from active pulmonary tuberculosis / H.C. Lai, Y.T. Horng, P.F. Yeh et al. // J. Microbiol. 2016 Nov; 54(11): 761-767. doi: 10.1007/s12275-016-6201-x.
35. Lishko V.K. Identification of Human Cathelicidin Peptide LL-37 as a Ligand for Macrophage Integrin αMβ2 (Mac-1, CD11b /CD18) that Promotes Phagocytosis by Opsonizing Bacteria / V.K. Lishko, B. Moreno, N.P. Podolnikova, T.P. Ugarova // Res. Rep. Biochem. 2016 Jul 7; 2016(6): 39-55. PMID: 27990411.
36. Mahlapuu M. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents / M. Mahlapuu, J. Håkansson, L. Ringstad, C. Björn // Front. Cell. Infect. Microbiol. 2016 Dec 27; 6: 194. doi: 10.3389/fcimb.2016.00194.
37. Midorikawa K. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes / K. Midorikawa, K. Ouhara, H. Komatsuzawa et al. // Infect. Immun. 2003 Jul; 71(7): 3730-9. doi: 10.1128/IAI.71.7.3730-3739.2003.
38. Montreekachon P. Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts / P. Montreekachon, P. Chotjumlong, J.G. Bolscher et al. // J. Periodontal Res. 2011 Jun; 46(3): 327-37. doi: 10.1111/j.1600-0765.2011.01346.x.
39. Neumann A. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps / A. Neumann, E.T. Berends, A. Nerlich et al. // Biochem J. 2014 Nov 15; 464(1): 3-11. doi: 10.1042 /BJ20140778.
40. Nijnik A., Hancock R.E. The roles of cathelicidin LL-37 in immune defences and novel clinical applications // Curr. Opin. Hematol. 2009 Jan; 16(1): 41-7.
41. Overhage J. Human host defense peptide LL-37 prevents bacterial biofilm formation / J. Overhage, A. Campisano, M. Bains et al. // Infect Immun. 2008 Sep; 76(9): 4176-82. doi: 10.1128/IAI.00318-08.].
42. Punde T.H. A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation / T.H. Punde, W.H. Wu, P.C. Lien et al. // Integr. Biol. (Camb). 2015 Feb; 7(2): 162-9. doi: 10.1039/c4ib00239c.
43. Ravensdale J. Efficacy of Antibacterial Peptides Against Peptide-Resistant MRSA Is Restored by Permeabilization of Bacteria Membranes / J. Ravensdale, Z. Wong, F. O’Brien, K. Gregg // Front. Microbiol. 2016 Nov 8; 7: 1745. doi: 10.3389/fmicb.2016.01745.
44. Rivas-Santiago B. Expression of cathelicidin LL-37 du–ring Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells / B. Rivas-San–tiago, R. Hernandez-Pando, C. Carranza et al. // Infect. Immun. 2008 Mar; 76(3): 935-41. doi: 10.1128/IAI.01218-07.
45. Rose C.E. Jr, Sung S.S., Fu S.M. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung // Microcirculation. 2003 Jun; 10(3-4): 273-88. doi: 10.1038/sj.mn.7800193.
46. Schrumpf J.A. Pro-inflammatory Cytokines Impair Vitamin D-induced Host Defense in Cultured Airway Epithelial Cells / J.A. Schrumpf, G.D. Amatngalim, J.B. Veldkamp et al. // Am. J. Respir. Cell. Mol. Biol. 2017 Feb 23. doi: 10.1165/rcmb.2016-0289OC.
47. Scott M.G. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses / M.G. Scott, D.J. Davidson, M.R. Gold et al. // J. Immunol. 2002 Oct 1; 169(7): 3883-91. doi: 10.4049/jimmunol.169.7.3883.
48. Seiler F. Regulation and function of antimicrobial peptides in immunity and diseases of the lung / F. Seiler, P.M. Lepper, R. Bals, C. Beisswenger // Protein Pept. Lett. 2014 Apr; 21(4): 341-51. doi: 10.2174/09298665113206660100.
49. Sun J. The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils-implications for asthma / J. Sun, B. Dahlén, B. Agerberth, J.Z. Haeggström // Allergy. 2013 Mar; 68(3): 304-11. doi: 10.1111/all.12087.
50. Tang X. P2X7 Receptor Regulates Internalization of Antimicrobial Peptide LL-37 by Human Macrophages That Promotes Intracellular Pathogen Clearance / X. Tang, D. Basavarajappa, J.Z. Haeggström, M. Wan // J. Immunol. 2015 Aug 1; 195(3): 1191-201. doi: 10.4049/jimmunol.1402845.
51. Tecle T., Tripathi S., Hartshorn K.L. Review: Defensins and cathelicidins in lung immunity // Innate Immun. 2010 Jun; 16(3): 151-9. doi: 10.1177/1753425910365734.
52. Tsou Y.A. Investigation of anti-infection mechanism of lactoferricin and splunc-1 / Y.A. Tsou, H.J. Huang, W.W. Lin, C.Y. Chen // Evid. Based Complement. Alternat. Med. 2014; 2014: 907028. doi: 10.1155/2014/907028.
53. Van der Does A.M. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature / A.M. van der Does, H. Beekhuizen, B. Ravensbergen et al. // J. Immunol. 2010 Aug 1; 185(3): 1442-9. doi: 10.4049/jimmunol.1000376.
54. Verjans E.T. Molecular mechanisms of LL-37-induced receptor activation: An overview / E.T. Verjans, S. Zels, W. Luyten et al. // Peptides. 2016 Nov; 85: 16-26. doi: 10.1016/j.peptides.2016.09.002.
55. Wan M. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages / M. Wan, van der Does A.M., X. Tang et al. // J. Leukoc Biol. 2014 Jun; 95(6): 971-81. doi: 10.1189/jlb.0513304.
56. Wan M. Cathelicidin LL-37 induces time-resolved release of LTB4 and TXA2 by human macrophages and triggers eicosanoid gene–ration in vivo / M. Wan, O. Soehnlein, X. Tang et al. // FASEB J. 2014 Aug; 28(8): 3456-67. doi: 10.1096/fj.14-251306.
57. Wang G. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments / G. Wang, B. Mishra, R.F. Epand, R.M. Epand // Biochim. Biophys. Acta. 2014 Sep; 1838(9): 2160-72. doi: 10.1016/j.bbamem.2014.01.016.
58. Wang X.Y. A Multiple Antigenic Peptide Mimicking Peptidoglycan Induced T Cell Responses to Protect Mice from Systemic Infection with Staphylococcus aureus / X.Y. Wang, Z.X. Huang, Y.G. Chen et al. // PLoS One. 2015 Aug 28; 10(8): e0136888. doi: 10.1371/journal.pone.0136888. eCollection 2015.
59. Wolf A.J. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria / A.J. Wolf, A. Arruda, C.N. Reyes et al. // J. Immunol. 2011 Dec 1; 187(11): 6002-10. doi: 10.4049/jimmunol.1100232.
60. Wu H. The Origin, Expression, Function and Future Research Focus of a G Protein-coupled Receptor, Mas-related Gene X2 (MrgX2) / H. Wu, M. Zeng, E.Y. Cho, W. Jiang, O. Sha // Prog. Histochem. Cytochem. 2015 Jul; 50(1-2): 11-7. doi: 10.1016/j.proghi.2015.06.001.
61. Yamaguchi Y., Ouchi Y. Antimicrobial peptide defensin: identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases // Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2012; 88(4): 152-66. doi: 10.2183/pjab.88.152.
62. Zhang Y. The human Cathelicidin LL-37 induces MUC5AC mucin production by airway epithelial cells via TACE-TGF-α-EGFR pathway / Y. Zhang, M. Zhu, Z. Yang et al. // Exp. Lung. Res. 2014 Sep; 40(7): 333-42. doi: 10.3109/01902148.2014.926434.
63. Zhang Z., Cherryholmes G., Shively J.E. Neutrophil secon–dary necrosis is induced by LL-37 derived from cathelicidin // J. Leukoc. Biol. 2008 Sep; 84(3): 780-8. doi: 10.1189/jlb.0208086.
64. Zurek O.W., Pallister K.B., Voyich J.M. Staphylococcus aureus Inhibits Neutrophil-derived IL-8 to Promote Cell Death // J. Infect. Dis. 2015 Sep 15; 212(6): 934-8. doi: 10.1093/infdis /jiv124.

/127-1.jpg )
/126-1.jpg )
/129-1.jpg )
/130-1.jpg )
/128-1.jpg )