αβТ-клетки адаптивной иммунной системы
В настоящее время существует широкий консенсус в отношении того, что αβТ-клетки адаптивной иммунной системы вносят существенный вклад в процесс патогенеза стафилококковой пневмонии, способствуя киллингу как вне-, так и внутриклеточно расположенных бактерий Staphylococcus aureus и участвуя в развитии воспаления. Т-лимфоциты способствуют как рекрутированию нейтрофилов и макрофагов в очаг поражения, так и синтезу высокоаффинных опсонизирующих антител, облегчающих опсонофагоцитоз внеклеточно расположенных бактерий Staphylococcus aureus. Продуцируемый Т-клетками IFN-γ участвует в элиминации бактерий, которые находятся внутри фагосом макрофагов. Цитотоксические Т-клетки лизируют инфицированные клетки с цитоплазматически расположенными патогенами и, высвобождая бактерии из внутриклеточного пространства, обеспечивают их доступность для механизмов фагоцитоза и киллинга. Также Т-клетки модулируют процесс воспаления: Тh1-, Тh17-клетки усиливают активность воспаления в начальной фазе, а Тreg-клетки ингибируют его в фазе реконвалесценции заболевания [9].
Конвенциональные αβТ-клетки представлены тремя субпопуляциями: CD4+-, CD8+Т-лимфоцитами и дважды отрицательными CD4–CD8–Т-клетками. В свою очередь, CD4+Т-лимфоциты организуют субпопуляции Th1-, –Th2-, Th9-, Th17-, Th22-, Treg- и TFH-клеток (табл. 1), а CD8+Т-лимфоциты — CD25, CD45RO, CD45RA, CCR-7, CD62L [21, 25, 50].
/171-1.jpg)
Наивные CD4+Т-клетки дифференцируются в различные субпопуляции эффекторных клеток, выполняющих специализированные функции. Микросреда определяет канализированность цитодифференцировки наивных CD4+Т-клеток. Цитодифференцировка наивных CD4+Т-клеток обусловлена: 1) в Th1-клетки — действием цитокинов IL-12 и IFN-γ, которые активируют факторы транскрипции STAT4 и STAT1, чтобы индуцировать T-bet и стимулировать продукцию IFN-γ соответственно; 2) в Th2-клетки — влиянием IL-4, который активирует STAT6, чтобы индуцировать GATA3 и cMaf; 3) в –Th17-клетки — действием TGFβ, IL-6 и –IL-23, которые через STAT3 и RORγt вызывают продукцию –IL-17A, IL-17F и IL-22 [64]. Также существует популяция эффекторных –CD4+T-клеток, обладающих признаками как Th1-, так и –Th17-клеток, — –Th1/17-клетки, которые экспрессируют факторы транскрипции RORγt, T-bet, хемокиновые рецепторы CXCR3, CCR6 и продуцируют цитокины IFN-γ, IL-17, IL-22. Человеческие Th1-, Th17-, –Th1/17-клетки играют важную роль в защите макроорганизма от различных патогенов [2, 6, 40].
Seng Jin Choi и соавт. [15] показали, что реакция CD4+Т-клеток является более эффективным механизмом защиты легочной ткани от бактерий Staphylococcus aureus, чем гуморальный иммунитет, опосредуемый В-клетками. Также продемонстрировано, что у пациентов с дефицитом Т-клеток отмечается высокий риск развития стафилококковой инфекции, а у мышей с дефицитом В-клеток уровень чувствительности к Staphylococcus aureus сопоставим с таковым у мышей дикого типа [65]. В связи с этим считают, что решающее значение в процессе саногенеза стафилококковой инфекции респираторного тракта играет Т-клеточно-опосредованный иммунитет, а не антистафилококковый антительный ответ. Julia B. Kolata и соавт. [32] продемонстрировали, что внеклеточные протеины бактерий Staphylococcus aureus индуцируют более активную клеточную реакцию, чем внутриклеточные стафилококковые белки. Авторы считают, что именно внеклеточные протеины бактерий Staphylococcus aureus представляют собой иммунодоминирующие стафилококковые антигены, активирующие Т-клетки. Существует три возможных объяснения данного факта: 1) у здоровых взрослых людей иммунная система в основном подвержена воздействию солютабных факторов, высвобождаемых бактериями Staphylococcus aureus; 2) при поглощении антигенпрезентирующими клетками внутриклеточные антигены бактерий Staphylococcus aureus недостаточно активно презентируются; 3) бактерии Staphylococcus aureus ингибируют Т-клеточную реакцию.
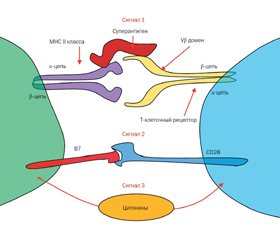
Активация Т-клеток может быть выполнена антигенами и суперантигенами бактерий Staphylococcus aureus (рис. 1).
Th1- и Th17-клетки
Особую роль в саногенезе стафилококковой пневмонии играют Th1- и Th17-клетки. Установлено, что антигенспецифические Th1- и Th17-клетки могут специфически взаимодействовать с рядом различных антигенов бактерий Staphylococcus aureus и способствовать рекрутингу нейтрофилов в очаг поражения до тех пор, пока сохраняются данные антигены [9].
Полагают, что рекрутирование фагоцитов, нейтрофилов и активация фагоцитоза для эффективной элиминации бактерий Staphylococcus aureus осуществляются при участии Th17-клеток, а бактерицидная активность фагоцитов регулируется преимущественно Th1-клетками за счет продукции IFN-γ [31, 35, 45]. Известно, что цитокин IFN-γ является мощным активатором моноцитов и предварительная обработка моноцитов цитокином IFN-γ резко увеличивает их потенциал внутриклеточного киллинга бактерий Staphylococcus aureus [22]. В настоящее время установлено, что, помимо своего типичного внеклеточного пребывания, бактерии Staphylococcus aureus способны сохраняться внутри клеток: эпителиоцитов, эндотелиоцитов, кератиноцитов, остеокластов, и даже внутри профессиональных фагоцитов (макрофагов и нейтрофилов). Внутриклеточно локализованные бактерии Staphylococcus aureus являются эндогенным резервуаром, выполняющим функцию источника реколонизации макроорганизма. Бактерии могут использовать гранулоциты в качестве троянских коней, облегчающих развитие абсцессов. Необходимо отметить, что IFN-γ продуцируется не только Th1-клетками, но и рядом других иммунных клеток: CD8+T-, γδT-, NK-клетками, ILC1 [9]. Цитокин IFN-γ активирует деятельность НАДФ-оксидазы, что способствует усилению АКМ-зависимого киллинга бактерий, расположенных внутри фагосомы макрофагов и нейтрофилов. Возбуждение рецептора IFN-γ в течение 15–30 мин активирует сигнальные пути Jak1 и Stat1, что приводит к транскрипции IFN-γ-чувствительных генов [61]. В отличие от многочисленных IFN-γ-чувствительных генов, характеризующихся быстрым транскрипционным ответом, активность генов, кодирующих компоненты NADPH-оксидазы в макрофагах человека, достигает максимума на 3-и — 4-е сутки после начала действия IFN-γ [13]. В макрофагах человека IFN-γ усиливает бактерицидную активность в течение первых 90 минут после фагоцитоза. Также IFN-γ усиливает связывание бактерий Staphylococcus aureus с макрофагами, но не влияет на их интернализацию [22].
Лица с генетическими заболеваниями, которые связаны с нарушениями Th17-ассоциированного сигнального пути, высоковосприимчивы к бактериям Staphylococcus aureus и грибам Candida albicans (табл. 2).
/172-1.jpg)
Продемонстрировано, что в культуре моноцитов и Т-клеток после инфицирования патогенами значительная часть пролиферирующих клеток приобретает способность продуцировать IL-17 и –IL-22 и экспрессировать RORγt и CCR6, являющиеся маркерами Th17-клеток. Первичный ответ Т-клеток in vitro на влияние бактерий Staphylococcus aureus и грибов Candida albicans приводит к дифференцировке Т-клеток в разные поляризованные типы T-клеток: Th1-клетки, продуцирующие исключительно IFN-γ; Th17-клетки, продуцирующие исключительно IL-17; и Th17-клетки, продуцирующие и IL-17, и IFN-γ. Интересно, что в клеточных культурах, инфицированных бактериями Staphylococcus aureus, большинство IL-17-секретирующих клеток не продуцируют IFN-γ и не экспрессируют фактор транскрипции T-bet, а в культуре клеток, инфицированных грибами Candida albicans, большинство IL-17-секретирующих клеток также продуцируют IFN-γ и экспрессируют RORγt и T-bet. На пятый день после рестимуляции Staphylococcus aureus антигенспецифические Th17-клетки, но не Candida albicans антигенспецифические клоны приобретают способность продуцировать IL-10. Необходимо отметить, что IL-1β in vitro и in vivo является мощным ингибитором продукции IL-10 Тh17-клетками памяти [69].
Treg-клетки
Бактерии Staphylococcus aureus могут стимулировать пролиферацию Th2- и Treg-клеток. Необходимо отметить, что, несмотря на известную способность Treg-клеток ограничивать поражение инфицированных тканей, подавляя активность воспалительного процесса, и снижать эффективность бактериального клиренса, способствуя хронизации инфекции, противовоспалительные эффекты, ассоциированные с Treg-клетками, у людей не изучались [9].
Т-клетки памяти
Инфицирование бактериями Staphylococcus aureus или вакцинация живыми бактериями способствует защите организма от последующего инфицирования Staphylococcus aureus. Предшествующее инфицирование бактериями Staphylococcus aureus способствует снижению бактериальной нагрузки Staphylococcus aureus приблизительно в 100 раз при последующей инфекции. Считают, что в основе антистафилококковой защиты лежит выраженное усиление продукции IFN-γ Staphylococcus aureus антигенспецифическими CD45RO+Th1-клетками памяти [12]. Barbara M. Bröker и Alex van Belkum [11] предположили, что здоровые особи также содержат большой пул специфичных к антигенам бактерий Staphylococcus aureus Т-клеток памяти. Julia B. Kolata и соавт. [32] было установлено, что у взрослых людей пул Staphylococcus aureus антигенспецифических T-клеток составляет около 3,6 % всех Т-клеток периферической крови, причем индивидуальное представительство данной антигенспецифической субпопуляции Т-клеток колеблется в широком диапазоне: от 0,2 до 5,7 %. Вероятно, солютабный внеклеточный субпротеом бактерий Staphylococcus aureus, который обогащен факторами вирулентности, содержит многочисленные стафилококковые антигены, способные взаимодействовать с TCR T-клеток, что и приводит к сильному IgG-ответу гуморального иммунитета у здоровых людей. Авторы предположили, что у здоровых лиц Staphylococcus aureus специфические Т-клетки памяти представляют значительный пул Т-клеток, численность которого устанавливается во время эпизодов субклинической инвазии стафилококковых бактерий. Среди специфичных к антигенам бактерий Staphylococcus aureus Т-клеток памяти обнаруживаются как Th1-, так и Th17-клетки [69].
При заражении бактериями Staphylococcus aureus в первую очередь реагируют антигенспецифические Т-клетки памяти. Вероятно, что их влияние на развитие воспалительного процесса превалирует над влиянием суперантигенреактивных Т-клеток. Стафилококковые суперантигены, как чрезвычайно мощные митогены Т-клеток, могут привести к развитию синдрома токсического шока. Однако это угрожающее жизни состояние встречается значительно реже, чем минорные эпизоды стафилококковой инвазии, постоянно происходящие у людей в повседневной жизни. Суперантигены обходят конвенциональный антигенный процессинг и легко нейтрализуются сывороточными специфическими антителами, присутствующими у большинства взрослых людей [39, 52], в то время как распознавание обычного стафилококкового антигена Т-клетками достаточно устойчиво к нейтрализующему действию антител. Специфическое связывание антител с белковыми антигенами не мешает взаимодействию антигенпрезентирующей клетки (antigen-presenting cells — АРС) с Т-клеткой, поскольку АРС способны эффективно обрабатывать и комплексы «антиген — антитело». Активация Staphylococcus aureus антигенспецифических T-клеток сопровождается высвобождением ими цитокинов, в основном с провоспалительными свойствами, и не ограничивается продукцией IL-1β, IL-17A и IFN-γ. Количество Т-клеток памяти, участвующих в реализации ответной реакции, и профиль цитокинового ответа, определяющий течение болезни, значительно различаются у разных индивидуумов и зависят от причинно-значимого штамма бактерий Staphylococcus aureus [32].
Бактерии Staphylococcus aureus для выживания в агрессивной среде макроорганизма при помощи своих токсинов ингибируют как специфические, так и неспецифические клеточные реакции иммунной системы (рис. 2).
В-клетки
При инфекционных заболеваниях активированные В-клетки выполняют разнообразные функции. В-лимфоциты обеспечивают защиту органов дыхания от инфекционных патогенных агентов. Считается, что продукция специфических антител плазматическими клетками объясняет высокую резистентность организма к большинству патогенных микроорганизмов. Однако установлено, что активированные В-клетки могут ингибировать механизмы защиты макроорганизма во время первичной инфекции, продуцируя противовоспалительные цитокины (IL-10 и IL-35) [20]. Необходимо отметить, что В-клетки, продуцирующие in vivo IL-10 и IL-35, относятся к различным субпопуляциям антителосекретирующих IgMhiCD19+CD138hi-клеток [20].
В настоящее время у мышей и людей выделено две основные субпопуляции В-лимфоцитов: В1- и В2-лимфоциты. B1-клетки развиваются из B1-предшественников в печени плода и сохраняются после рождения на протяжении всей жизни как самовозобновляемая субпопуляция, клетки которой практически не высвобождаются из костного мозга в течение постнатального периода жизни. Тогда как B2-лимфоциты развиваются из транзиторных B2-клеток, происходящих из предшественников, которые на протяжении всей жизни высвобождаются в периферическое русло крови из костного мозга. В свою очередь, группа субпопуляции В1-клеток состоит из CD5+CD11b–В1a-клеток, активно продуцирующих IgM, и CD5–CD11b+В1b-клеток, слабо продуцирующих IgM, а популяция В2-лимфоцитов — из маргинальных (MZ) и фолликулярных (FO) В-клеток [23, 27].
В1-клетки
Cубпопуляция В1-клеток состоит из
CD5+CD11b–В1a-клеток, активно продуцирующих IgM, и CD5–CD11b+В1b-клеток, слабо продуцирующих IgM [5, 23, 27].
Иммуноглобулинпродуцирующие В1-клетки пре–имущественно находятся в перитонеальной и плевральной полостях и продуцируют естественные антитела класса IgM против так называемых Т-независимых антигенов, обычно углеводных или фосфолипидных антигенов, характерных для комменсальных бактерий. Прототипом антител, секретируемых В1-клетками, являются антитела против антигенов АВО групп крови. Естественные антитела являются полиреактивными или полиспецифическими, поскольку они могут связываться как с аутоантигенами, так и с микробными антигенами [26, 37]. В1-клетки кроме IgM продуцируют полиреактивные IgA-антитела, которые вносят свой вклад в защиту слизистых оболочек наряду с IgA, секретируемым FO-B2-лимфоцитами [60]. Естественные антитела защищают макроорганизм от антигенов (например, капсульных полисахаридов), которые не вызывают монореактивный антительный ответ [17]. Так как естественные антитела постоянно присутствуют в сыворотке крови, антигены патогенных агентов, в частности бактерий Staphylococcus aureus, сразу после инфицирования взаимодействуют с данными антителами [16, 24]. Необходимо отметить, что CD5+CD11b–В1a-клетки представляют собой долгоживущие лимфоциты и их потеря может быть необратимой. Поверхностный протеин A (surface protein A — SpA) бактерий Staphylococcus aureus, являясь В-клеточным суперантигеном, вызывает перекрестное сшивание рецепторов и индуцирует гибель определенных B-клеток, нарушая полноту репертуара естественных антистафилококковых антител. Данный антительный дефект может быть обнаружен в течение года после контакта с SpA [58].
В2-клетки и гуморальный ответ
Cубпопуляция В2-лимфоцитов состоит из маргинальных (MZ) и фолликулярных (FO) В-клеток [23, 27].
Транзиторные B2-клетки после индукции протеина 2 гомолога notch (notch homolog protein 2 — NOTCH2) дифференцируются в MZ-B-клетки. Данные клетки экспрессируют полиреактивный B-клеточный рецептор (B cell receptor — BCR), рецепторы комплемента (CD21 и CD35) и молекулу CD1d, подобную молекулам МНС I класса. MZ-B-клетки продуцируют полиреактивные IgM-антитела, которые облегчают элиминацию микроорганизмов и апоптотических клеток [14]. Подобно В1-клеткам MZ-В-клетки распознают Т-независимые углеводные, фосфолипидные антигены и без помощи Т-клеток пролиферируют и секретируют низкоаффинные антитела (IgM). В отличие от B1-клеток –MZ-B-клетки участвуют в реакциях и на T-зависимые белковые антигены, генерируя высокоаффинные антитела [3]. Как B1-, так и –MZ-B-клетки на внедрение инфекционного патогена реагируют быстрым (приблизительно через 1–3 суток) IgM-антительным ответом. Таким образом, MZ-B-клетки представляют собой универсальную популяцию клеток, которые быстро вырабатывают антитела на T-независимые и T-зависимые антигены [14].
Протеин SpA бактерий Staphylococcus aureus также истощает субпопуляцию MZ-B-клеток, снижая уровень ранней защиты от данных бактерий [70].
Фолликулярные B-клетки развиваются в селезенке из транзиторных B2-клеток после BCR-опосредованной индукции Bruton тирозинкиназы и локализуются в лимфатических узлах и селезенке. Эти В-клетки являются конвенциональными В-лимфоцитами адаптивной иммунной системы и представляют самую многочисленную субпопуляцию В-клеток. Основной функцией FO-B-клеток является синтез долгоживущих высокоаффинных IgG-антител против Т-зависимых антигенов [28].
После того как В-клетки выходят из эндотелиальных венул, они мигрируют в фолликулярную зону лимфоузла. Фолликулярные В-клетки, связавшись с родственным антигеном, мигрируют: 1) к границе фолликула, где они взаимодействуют с T-хелперами; или 2) в межфолликулярное пространство и дифференцируются в клетки, обладающие способностью к ранней секреции антител; или 3) мигрируют в герминальный (зародышевый) центр (ГЦ) [1, 44].
Формирование продуктивного гуморального ответа в ГЦ лимфоузлов зависит от межклеточных взаимодействий FO-B-клеток. Различают четыре сигнала, активирующие В-клетки: сигнал 1, ассоциированный с BCR, который распознает антигены микроорганизма; сигнал 2, обусловленный непосредственным физическим взаимодействием –FO-B-клеток с Т-клетками; сигнал 3, связанный с активацией патогенассоциированными молекулярными структурами TLR В-клеток; и сигнал 4, вызванный действием цитокинов, секретируемых клетками, расположенными в непосредственной близости [20, 53]. Различают два варианта активации В-клеток: зависимый и независимый от Т-клеток. Активация FO-B-клеток, зависимая от Т-клеток, обычно происходит в ГЦ лимфатического узла. Герминальный центр представляет собой интенсивное содружество клеток: GC-, TFH-, T-фолликулярных регуляторных (TFR) клеток, макрофагов твитильного тела и фолликулярных дендритных клеток (follicular dendritic cells — FDC). Стимуляция антигеном BCR вызывает потребность в костимуляции, обеспечиваемой CD4+TFH-клетками. В отсутствие Т-хелперной костимуляции В-клетки погибают [51].
Первоначально TFH-клетки были выделены в фолликулах ГЦ как CD4+Т-клетки памяти, ассоциированные с В-клетками и экспрессирующие хемокиновый рецептор CXCR5 [55]. Хелперные TFH-клетки в ГЦ являются основным источником IL-21, который играет решающую роль в переключении иммуноглобулиновых классов при синтезе антител [49]. Также IL-21 способствует дифференцировке FO-B-клеток в плазматические антителопродуцирующие клетки и определяет выживание В-клеток памяти в ГЦ [34, 48].
В отличие от других Т-хелперных линий TFH-клетки экспрессируют репрессор транскрипции Bcl6. Также TFH-клетки экспрессируют рецепторы и костимулирующие молекулы, которые взаимодействуют с их родственными лигандами на FO-B-, Т-клетках и DC (табл. 3) [54].
В настоящее время в периферической крови идентифицированы CD4+T-клетки, высоко экспрессирующие CXCR5 и проявляющие функции, подобные TFH-клеткам, обнаруженным в лимфатических узлах. TFH-клетки периферической крови секретируют высокие уровни IL-21 и способны взаимодействовать с В-клетками [42]. Также описаны IL-21-секретирующие и фенотипически подобные TFH-клеткам лимфоузлов Т-клетки периферической крови: PD-1+CXCR3–CXCR5+CD4+Т-клетки памяти [38], CCR7hiCXCR5hiCCR6hiPD-1hiCD4+T-клетки [8].
Перед взаимодействием с В-клетками T-хелперы активируются АРС. Взаимодействие Т- и В-клеток через CD40L (на Т-клетках) и CD40 (на В-клетках) требуется для дифференцировки В-клеток, в том числе для переключения синтеза иммуноглобулиновых классов с IgM на IgG/IgA/IgE и соматической гипермутации (созревания аффинности антител) (рис. 3) [33, 56].
Активация В-клеток независимо от Т-лимфоцитов осуществляется при взаимодействии BCR и TLR с продуктами бактерий Staphylococcus aureus или с цитокинами. Одновременной стимуляции BCR и TLR достаточно, чтобы вызвать пролиферацию В-клеток, но недостаточно для индукции стимуляции рекомбинации и соматической гипермутации (рис. 4) [20, 27].
/178-1.jpg)
Продукция антител, специфических к антигенам бактерий Staphylococcus aureus, может происходить и в экстрафолликулярном регионе. Экстрафолликулярный антительный ответ характерен для ранней стадии инфекционного процесса и может осуществляться с помощью или без помощи Т-клеток [19]. Экстрафолликулярные плазматические клетки являются короткоживущими клетками и не обес–печивают долгосрочной продукции антибактериальных антител. Также Amanda B. Keener и соавт. [30] было показано, что протеин SpA Staphylococcus aureus изменяет судьбу плазмокластов и плазматических клеток, способствуя повышению уровня выживания экстрафолликулярных короткоживущих плазматических клеток и сокращению пула долгоживущих плазматических клеток. Отсутствие долгоживущих плазматических клеток ассоциируется с быстрым снижением титра антигенспецифических антистафилококковых антител. Влияние протеина SpA на плазматические клетки ассоци–ировано только со вторичным иммунным ответом, поскольку в течение первичного ответа активность антителогенеза и формирование В-клеток памяти у мышей не зависели от наличия протеина SpA у штаммов Staphylococcus aureus. В ГЦ FO-B-клетки пролиферируют и дифференцируются в долгоживущие плазматические клетки или В-клетки памяти. Плазматические клетки синтезируют низкоаффинные антитела с неизмененным не прошедшим соматической гипермутации антигенсвязывающим регионом. Процесс переключения изотипов антител происходит как в экстрафолликулярном регионе, так и в ГЦ, регулируется TFH-клетками [1].
Результаты исследований свидетельствуют о том, что колонизация бактериями Staphylococcus aureus эпителия респираторного тракта недостаточна, чтобы вызвать специфический IgG-ответ [41]. Более того, экспериментальная колонизация носовой полости у людей не вызывает устойчивого IgG-ответа [28]. Silva Holtfreter и соавт. [29] считают, что специ–фическая иммунная память к Staphylococcus aureus, наблюдаемая у большинства взрослых людей, вызвана небольшими инвазивными эпизодами.
Антитела являются гуморальными эффекторными молекулами адаптивной иммунной системы. Нейтрализующие антитела связываются с токсинами, высвобождаемыми бактериями Staphylococcus aureus, и препятствуют их действию [4, 7]. Также антитела, антигенспецифически прикрепляясь к поверхности бактерий, индуцируют классический путь комплементзависимого киллинга бактерий Staphylococcus aureus и способствуют их фагоцитозу. Однако необходимо отметить, что бактерии Staphylococcus aureus высокорезистентны к комплементзависимому лизису [10]. Кроме того, антитела могут опсонировать бактерии, способствуя фагоцитозу, и нейтрализовывать факторы вирулентности. Специфические антитела являются маркерами адаптивного ответа памяти, в котором принимают участие Staphylococcus aureus реактивные Т-клетки памяти [32].
Специфические антитела к бактериям Staphylococcus aureus препятствуют развитию инфекционного процесса, и у большинства людей концентрация их достаточна для проявления протекционного эффекта. Установлено, что в сыворотке крови новорожденных непосредственно после родов содержатся только материнские антистафилококковые IgG-антитела, титр которых постепенно, с увеличением возраста ребенка, снижается и достигает минимума к шести месяцам жизни. У детей именно в этом возрасте появляются собственные антистафилококковые антитела иммуноглобулинового класса M, а затем A- и G-класса [66]. У взрослых людей отмечается наличие разных иммуноглобулиновых композиций сывороточных антител, направленных против широкого спектра антигенов бактерий Staphylococcus aureus [9]. Представляет особый интерес тот факт, что в сыворотке крови лиц, являющихся и не являющихся носителями бактерий Staphylococcus aureus, наблюдается высокий титр антистафилококковых антител против консервативных стафилококковых антигенов. Высокий уровень антистафилококковых антител у лиц, не являющихся носителями бактерий Staphylococcus aureus, свидетельствует о том, что они прежде подвергались стафилококковому инфицированию. Лица с носительством бактерий Staphylococcus aureus отличаются наличием повторных эпизодов инфекции, вызванных колонизирующим штаммом, что приводит к развитию сильного штаммоспецифического иммунного ответа. В отличие от носителей лица без носительства бактерий Staphylococcus aureus, контактируя с широким спектром различных его изолятов, характеризуются наличием антительного ответа к различным штаммам Staphylococcus aureus. Таким образом, клинически здоровые носители бактерий Staphylococcus aureus характеризуются устойчивым антистафилококковым штаммоспецифическим антительным ответом, а неносители — антистафилококковым штаммонеспецифическим антительным ответом. Однако необходимо отметить, что наличие антистафилококковых антител не гарантирует абсолютного предупреждения возникновения новых эпизодов стафилококковой инфекции и полной элиминации бактерий Staphylococcus aureus [29].
Развитие инфекционного процесса, вызванного бактериями Staphylococcus aureus, у иммунокомпетентных индивидуумов сопровождается выраженным антителообразованием [9]. Исследование титра антител к Staphylococcus aureus продемонстрировало, что наиболее высокий титр антител против стафилококковых антигенов наблюдается у колонизированных субъектов [65]. В то же время у лиц с носительством бактерий Staphylococcus aureus чаще отмечается возникновение бактериемии, чем у лиц без носительства [68]. Sebastian Stentzel и соавт. [59] продемонстрировали, что у 95–100 % пациентов с бактериемией IgG-антительный ответ индуцируется в отношении нескольких стафилококковых антигенов, включая иммунные модуляторы: стафилококкового суперантигенподобного протеина 3 (staphylococcal superantigen-like protein 3 — SSL3), SSL10, протеина уклонения от иммунного ответа SCIN (staphylococcal complement inhibitor), γ-гемолизина (Hlγ), лейкоцидина F (LukF), АВС транспортера SA0688, мембранно-ассоциированной фолдазы PrsA и связанного с поверхностью мембраны и внеклеточного энзима IsaA [18].
В зависимости от пути инфицирования бактериями Staphylococcus aureus генерируется различный спектр разного титра антител. При внутривенном инфицировании у мышей наблюдается наиболее выраженный антительный ответ с генерацией широкого спектра специфических антител. В частности, при внутривенном инфицировании отмечается более выраженный ответ на такие факторы вирулентности, как γ-гемолизин, лейкоцидин PV, сериновые протеазы SplB, SplD и α-токсин (Hlα), чем при внутримышечном инфицировании. Представляет интерес тот факт, что внутримышечное инфицирование не защищает экспериментальных животных от стафилококковой реинфекции. Показано, что только внутривенное, но не внутримышечное введение стафилококковых бактерий защищает экспериментальных мышей от последующего инфицирования высокими дозами бактерий Staphylococcus aureus [57].
При инфицировании бактериями Staphylococcus aureus легких у мышей достоверно повышаются уровни концентрации IgG-антител, направленных против Nuc, IsaA, Efb, α-токсина, LukE, LukS и SSL1. У мышей при инфицировании легких наблюдается более высокий титр IgG-антител, направленных против IsdA, FnbpB, SCIN, HlgB, LukF, TSST-1, SSL5 и SSL9, чем у мышей, которые инфицированы внутримышечным путем введения бактерий Staphylococcus aureus [64].
Уровень концентрации антистафилококковых антител коррелирует с тяжестью и вероятностью прогрессирования заболевания. Пациенты с благоприятным исходом заболевания характеризуются более высокими уровнями антител, направленных против внеклеточных ферментов IsaA, Plc и GlpQ, а также гомолога Eap EapH2 (SACOL0985) бактерий Staphylococcus aureus [59].
Martina Selle и соавт. [57] установили, что стафилококковые антигены SACOL0985 (EapH2), SACOL1788, SACOL0021 и SACOL0129 являются наиболее перспективными антигенами, которые могут быть использованы для разработки эффективной противостафилококковой вакцины.
Согласно данным доклинических исследований, пассивная иммунизация с использованием противостафилококковых антител способствует выздоровлению пациентов и экспериментальных животных, страдающих стафилококковой инфекцией [43]. Пассивный перенос антител, направленных против различных стафилококковых антигенов (например, ClfA, Hla, IsdA, IsdB, FhuD2), обеспечивает защиту от бактерий Staphylococcus aureus у экспериментальных животных. Результаты исследований in vitro показали, что антитела могут играть непосредственную роль в ингибировании функции факторов вирулентности и токсинов. Например, антитела могут нейтрализовать токсичность Hla, предупреждать захват ионов железа, опосредуемого IsdB и IsdA, или ингибировать FhuD2-зависимый рост бактерий [47]. Однако при стафилококковой инфекции гуморальный ответ иммунной системы может быть подавлен некоторыми факторами бактерий Staphylococcus aureus [62]. Так, например, SpA и Sbi блокируют циркулирующие антитела, SpA ингибирует развитие гуморального ответа, вызывая истощение B-клеток [47].
Необходимо отметить, что ни одна из разработанных стафилококковых вакцин не была рекомендована для клинического применения [67]. В настоящее время установлено, что при стафилококковой инфекции антительный ответ не предупреждает летальный исход заболевания.
Таким образом, бактерии Staphylococcus aureus представляют собой патогенные микроорганизмы, инфицирование которыми может проявляться в виде двух противоположных клинических форм. С одной стороны, часть (около половины) взрослого населения устойчиво колонизирована бактериями Staphylococcus aureus без манифестации клинических симптомов, с другой стороны, бактерии Staphylococcus aureus у некоторых людей вызывают тяжело протекающие инфекции, резистентные к антибактериальной терапии [29].
Бактерии Staphylococcus aureus продуцируют поверхностные белки, одни из которых участвуют в колонизации дыхательных путей, не вызывая защитного иммунного ответа, а другие при попадании в нижние дыхательные пути, активируя образраспознающие рецепторы эпителиоцитов и иммуноцитов, индуцируют воспалительный процесс. Внеклеточные стафилококковые бактерии элиминируются при помощи макрофагов и нейтрофилов. Внутриклеточные стафилококковые бактерии элиминируются после гибели инфицированной клетки, которая осуществляется различными механизмами, в том числе и специфическими цитотоксическими Т-лимфоцитами. Механизмы врожденной и адаптивной иммунной системы при стафилококковой инфекции не достигают полной элиминации возбудителя. По мнению Barbara M. Bröker [9], физиологическая задача адаптивной иммунной системы, вероятно, заключается в контроле роста колонии бактерий Staphylococcus aureus и в быстром восстановлении равновесия отношения макро- и микроорганизма. Авторы считают, что реальной целью терапии стафилококковой инфекции является не тотальная элиминация бактерий Staphylococcus aureus, а предупреждение развития опасных инвазивных осложнений.
Конфликт интересов. Авторы заявляют об отсутствии какого-либо конфликта интересов при подготовке данной статьи.
Список литературы
1. Топтыгина А.П. Лимфоидный фолликул — территория иммунного ответа // Иммунология. 2012; № 3 http://cyberleninka.ru/article/n/limfoidnyy-follikul-territoriya-immunnogo-otveta.
2. Annunziato F. Phenotypic and functional features of human Th17 cells / F. Annunziato, L. Cosmi, V. Santarlasci et al. // J. Exp. Med. 2007 Aug 6; 204(8): 1849-61. doi: 10.1084/jem.20070663.
3. Arnon T.I. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress / T.I. Arnon, R.M. Horton, I.L. Grigo–rova, J.G. Cyster // Nature. 2013 Jan 31; 493(7434): 684-8. doi: 10.1038/nature11738.
4. Badarau A. Staphylococcus aureus for the generation of neutralizing antibodies / A. Badarau, H. Rouha, S. Malafa et al. // MAbs. 2016 Oct; 8(7): 1347-1360. doi: 10.1080/19420862.2016.1215791.
5. Baumgarth N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production // Front Immunol. 2016 Sep 9; 7: 324. doi: 10.3389/fimmu.2016.00324.
6. Becattini S. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines / S. Becattini, D. Latorre, F. Mele et al. // Science. 2015 Jan 23; 347(6220): 400-6. doi: 10.1126/science.1260668.
7. Berube B.J., Bubeck Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue // Toxins (Basel). 2013 Jun; 5(6): 1140-66. PMID: 23888516.
8. Boswell K.L. Loss of circulating CD4 T cells with B cell hel–per function during chronic HIV infection / K.L. Boswell, R. Paris, E. Boritz et al. // PLoS Pathog. 2014 Jan 30; 10(1): e1003853. doi: 10.1371/journal.ppat.1003853.
9. Bröker B.M. The T Cell Response to Staphylococcus aureus / B.M. Bröker, D. Mrochen, V. Péton et al. // Pathogens. 2016 Mar 17; 5(1). pii: E31. doi: 10.3390/pathogens5010031.
10. Bröker B.M., Holtfreter S., Bekeredjian-Ding I. Immune control of Staphylococcus aureus — regulation and counter-regulation of the adaptive immune response // Int. J. Med. Microbiol. 2014 Mar; 304(2): 204-14. doi: 10.1016/j.ijmm.2013.11.008.
11. Bröker B.M., van Belkum A. Immune proteomics of Staphylococcus aureus // Proteomics. 2011 Aug; 11(15): 3221-31. doi: 10.1002/pmic.201100010.
12. Brown A.F. Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection / A.F. Brown, A.G. Murphy, S.J. Lalor et al. // PLoS Pathog. 2015 Nov 5; 11(11): e1005226. doi: 10.1371/journal.ppat.1005226.
13. Casbon A.J. Effects of IFN-γ on intracellular trafficking and activity of macrophage NADPH oxidase flavocytochrome b558 / A.J. Casbon, M.E. Long, K.W. Dunn et al. // J. Leukoc Biol. 2012 Oct; 92(4): 869-82. doi: 10.1189/jlb.0512244.
14. Cerutti A., Cols M., Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes // Nat. Rev. Immunol. 2013 Feb; 13(2): 118-32. doi: 10.1038/nri3383.
15. Choi S.J. Active Immunization with Extracellular Vesicles Derived from Staphylococcus aureus Effectively Protects against Staphylococcal Lung Infections, Mainly via Th1 Cell-Mediated Immunity / S.J. Choi, M.H. Kim, J. Jeon et al. // PLoS One. 2015 Sep 2; 10(9): e0136021. doi: 10.1371/journal.pone.0136021.
16. Cohen T.S. Staphylococcus aureus α toxin potentiates opportunistic bacterial lung infections / T.S. Cohen, J.J. Hilliard, O. Jones-Nelson et al. // Sci Transl. Med. 2016 Mar 9; 8(329): 329ra31. doi: 10.1126/scitranslmed.aad9922.
17. Cunningham A.F. B1b cells recognize protective antigens after natural infection and vaccination / A.F. Cunningham, A. Flores-Langarica, S. Bobat et al. // Front Immunol. 2014 Oct 31; 5: 535. doi: 10.3389/fimmu.2014.00535.
18. Den Reijer P.M. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood / P.M. den Reijer, N. Lemmens-den Toom, S. Kant et al. // PLoS One. 2013; 8(1): e53391. doi: 10.1371/journal.pone.0053391.
19. Feldman S. Chronic airway inflammation provides a unique environment for B cell activation and antibody production / S. Feldman, R. Kasjanski, J. Poposki et al. // Clin. Exp. Allergy. 2016 Dec 21. doi: 10.1111/cea.12878.
20. Fillatreau S. Regulatory roles of B cells in infectious disea–ses // Clin. Exp. Rheumatol. 2016 Jul-Aug; 34(4 Suppl 98): PMID: 27586794.
21. Golubovskaya V., Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy // Cancers (Basel). 2016 Mar 15; 8(3). pii: E36. doi: 10.3390/cancers8030036.
22. Greenlee-Wacker M.C., Nauseef W.M. IFN-γ targets macrophage-mediated immune responses toward Staphylococcus aureus // J. Leukoc Biol. 2017 Mar; 101(3): 751-758. doi: 10.1189/jlb.4A1215-565RR.
23. Griffin D.O., Rothstein T.L. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus // J. Exp. Med. 2011 Dec 19; 208(13): 2591-8. doi: 10.1084/jem.20110978.
24. Gunti S., Notkins A.L. Polyreactive Antibodies: Function and Quantification // J. Infect. Dis. 2015 Jul 15; 212 Suppl 1: S42-6. doi: 10.1093/infdis/jiu512.
25. Hale J.S., Ahmed R. Memory T follicular helper CD4 T cells // Front Immunol. 2015 Feb 2; 6: 16. doi: 10.3389/fimmu.2015.00016.
26. Hardy R.R., Hayakawa K. Selection of natural autoreactive B cells // Clin. Exp. Rheumatol. 2015 Jul-Aug; 33(4 Suppl 92): S80-6.
27. Hoffman W., Lakkis F.G., Chalasani G. B Cells, Antibodies, and More // Clin. J. Am. Soc. Nephrol. 2016 Jan 7; 11(1): 137-54. doi: 10.2215/CJN.09430915.
28. Holtfreter S. Human immune proteome in experimental colonization with Staphylococcus aureus / S. Holtfreter, T.T. Nguyen, H. Wertheim et al. // Clin. Vaccine Immunol. 2009 Nov; 16(11): 1607-14. doi: 10.1128/CVI.00263-09.
29. Holtfreter S. Omics Approaches for the Study of Adaptive Immunity to Staphylococcus aureus and the Selection of Vaccine Candidates / S. Holtfreter, J. Kolata, S. Stentzel et al. // Proteomes. 2016 Mar 7; 4(1). pii: E11. doi: 10.3390/proteomes4010011.
30. Keener A.B. Staphylococcus aureus Protein A Disrupts Immunity Mediated by Long-Lived Plasma Cells / A.B. Keener, L.T. Thurlow, S. Kang et al. // J. Immunol. 2017 Feb 1; 198(3): 1263-1273. doi: 10.4049/jimmunol.1600093.
31. Kim M.R. Staphylococcus aureus-derived extracellular ve–sicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses / M.R. Kim, S.W. Hong, E.B. Choi et al. // Allergy. 2012 Oct; 67(10): 1271-81. doi: 10.1111/all.12001.
32. Kolata J.B. The Fall of a Dogma? Unexpected High T-Cell Memory Response to Staphylococcus aureus in Humans / J.B. Kolata, I. Kühbandner, C. Link et al. // J. Infect. Dis. 2015 Sep 1; 212(5): 830-8. doi: 10.1093/infdis/jiv128.
33. Krishna S., Miller L.S. Innate and adaptive immune respon–ses against Staphylococcus aureus skin infections // Semin Immunopathol. 2012 Mar; 34(2): 261-80. doi: 10.1007/s00281-011-0292-6.
34. Leonard W.J., Wan C.K. IL-21 Signaling in Immunity // F1000Res. 2016 Feb 26; 5. pii: F1000 Faculty Rev-224. doi: 10.12688/f1000research.7634.1.
35. Lin L. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice / L. Lin, A.S. Ibrahim, X. Xu et al. // PLoS Pathog. 2009 Dec; 5(12): e1000703. doi: 10.1371/journal.ppat.1000703.
36. Linterman M.A., Hill D.L. Can follicular helper T cells be targeted to improve vaccine efficacy? // F1000Res. 2016 Jan 20; 5. pii: F1000 Faculty Rev-88. doi: 10.12688/f1000research.7388.1.
37. Lobo P.I. Role of Natural Autoantibodies and Natural IgM Anti-Leucocyte Autoantibodies in Health and Disease // Front Immunol. 2016 Jun 6; 7: 198. doi: 10.3389/fimmu.2016.00198.
38. Locci M. Human circulating PD-1+CXCR3-CXCR5+ me–mory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses / M. Locci, C. Havenar-Daughton, E. Landais et al. // Immunity. 2013 Oct 17; 39(4): 758-69. doi: 10.1016/j.immuni.2013.08.031.
39. Lönnqvist A. Neonatal exposure to staphylococcal superantigen improves induction of oral tolerance in a mouse model of airway allergy / A. Lönnqvist, S. Ostman, N. Almqvist et al. // Eur. J. Immunol. 2009 Feb; 39(2): 447-56. doi: 10.1002/eji.200838418.
40. Ma C.S. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets / C.S. Ma, N. Wong, G. Rao et al. // J. Exp. Med. 2016 Jul 25; 213(8): 1589-608. doi: 10.1084/jem.20151467.
41. Missiakas D., Schneewind O. Staphylococcus aureus vaccines: Deviating from the carol // J. Exp. Med. 2016 Aug 22; 213(9): 1645-53. doi: 10.1084/jem.20160569.
42. Morita R. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion / R. Morita, N. Schmitt, S.E. Bentebibel et al. // Immunity. 2011 Jan 28; 34(1): 108-21. doi: 10.1016/j.immuni.2010.12.012.
43. Otto M. Novel targeted immunotherapy approaches for staphy–lococcal infection // Expert Opin Biol. Ther. 2010 Jul; 10(7): 1049-59. doi: 10.1517/14712598.2010.495115.
44. Park C. Lymph node B lymphocyte trafficking is constrained by anatomy and highly dependent upon chemoattractant desensitization / C. Park, I.Y. Hwang, R.K. Sinha et al. // Blood. 2012 Jan 26; 119(4): 978-89. doi: 10.1182/blood-2011-06-364273.
45. Parker D. CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia / D. Parker, C.L. Ryan, F. Alonzo 3rd et al. // J. Infect. Dis. 2015 Mar 1; 211(5): 835-45. doi: 10.1093/infdis/jiu525.
46. Patel D.D., Kuchroo V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions // Immunity. 2015 Dec 15; 43(6): 1040-51. doi: 10.1016/j.immuni.2015.12.003.
47. Pozzi C. Phagocyte subsets and lymphocyte clonal deletion behind ineffective immune response to Staphylococcus aureus / C. Pozzi, G. Lofano, F. Mancini et al. // FEMS Microbiol. Rev. 2015 Sep; 39(5): 750-63. doi: 10.1093/femsre/fuv024.
48. Qi H. T follicular helper cells in space-time // Nat. Rev. Immunol. 2016 Oct; 16(10): 612-25. doi: 10.1038/nri.2016.94.
49. Rahe M.C., Murtaugh M.P. Interleukin-21 Drives Proli–feration and Differentiation of Porcine Memory B Cells into Antibody Secreting Cells // PLoS One. 2017 Jan 26; 12(1): e0171171. doi: 10.1371/journal.pone.0171171.
50. Raphael I. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases / I. Raphael, S. Nalawade, T.N. Eagar, T.G. Forsthuber // Cytokine. 2015 Jul; 74(1): 5-17. doi: 10.1016/j.cyto.2014.09.011.
51. Rettig T.A. Evasion and interactions of the humoral innate immune response in pathogen invasion, autoimmune disease, and cancer / T.A. Rettig, J.N. Harbin, A. Harrington et al. // Clin. Immunol. 2015 Oct; 160(2): 244-54. doi: 10.1016/j.clim.2015.06.012.
52. Rouha H. Five birds, one stone: neutralization of α-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody / H. Rouha, A. Badarau, Z.C. Visram et al. // MAbs. 2015; 7(1): 243-54. doi: 10.4161/19420862.2014.985132.
53. Sahay B. Activation of B cells by a dendritic cell-targeted oral vaccine / B. Sahay, J.L. Owen, T. Yang et al. // Curr. Pharm. Biotechnol. 2013; 14(10): 867-77. PMID: 24372255.
54. Sawaf M., Dumortier H., Monneaux F. Follicular Helper T Cells in Systemic Lupus Erythematosus: Why Should They Be Considered as Interesting Therapeutic Targets? // J. Immunol. Res. 2016; 2016: 5767106. doi: 10.1155/2016/5767106.
55. Schaerli P. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function / P. Schaerli, K. Willimann, A.B. Lang et al. // J. Exp. Med. 2000 Dec 4; 192(11): 1553-62. PMID: 11104798.
56. Seifert M., Küppers R. Human memory B cells // Leukemia. 2016 Dec; 30(12): 2283-2292. doi: 10.1038/leu.2016.226.
57. Selle M. Global antibody response to Staphylococcus aureus live-cell vaccination / M. Selle, T. Hertlein, B. Oesterreich et al. // Sci Rep. 2016 Apr 22; 6: 24754. doi: 10.1038/srep24754.
58. Silverman G.J., Goodyear C.S. Confounding B-cell defences: lessons from a staphylococcal superantigen // Nat. Rev. Immunol. 2006 Jun; 6(6): 465-75. doi: 10.1038/nri1853.
59. Stentzel S. Specific serum IgG at diagnosis of Staphylococcus aureus bloodstream invasion is correlated with disease progression / S. Stentzel, N. Sundaramoorthy, S. Michalik et al. // J. Proteomics. 2015 Oct 14; 128: 1-7. doi: 10.1016/j.jprot.2015.06.018.
60. Suzuki K. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity / K. Suzuki, M. Maruya, S. Kawamoto, S. Fagarasan // Immunol. Rev. 2010 Sep; 237(1): 180-90. doi: 10.1111/j.1600-065X.2010.00941.x.
61. Swindle E.J. Interferon-γ enhances both the anti-bacterial and the pro-inflammatory response of human mast cells to Staphylococcus aureus / E.J. Swindle, J.M. Brown, M. Rådinger et al. // Immunology. 2015 Nov; 146(3): 470-85. doi: 10.1111/imm.12524.
62. Thammavongsa V. Staphylococcal manipulation of host immune responses / V. Thammavongsa, H.K. Kim, D. Missiakas, O. Schneewind // Nat. Rev. Microbiol. 2015 Sep; 13(9): 529-43. doi: 10.1038/nrmicro3521.
63. Vahedi G. Helper T-cell identity and evolution of diffe–rential transcriptomes and epigenomes / G. Vahedi, A.C. Poholek, T.W. Hand et al. // Immunol. Rev. 2013 Mar; 252(1): 24-40. doi: 10.1111/imr.12037.
64. Van den Berg S. A multiplex assay for the quantification of antibody responses in Staphylococcus aureus infections in mice / S. van den Berg, M.G. Bowden, T. Bosma et al. // J. Immunol. Methods. 2011 Feb 28; 365(1-2): 142-8. doi: 10.1016/j.jim.2010.12.013.
65. Verkaik N.J. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus / N.J. Verkaik, C.P. de Vogel, H.A. Boelens et al. // J. Infect. Dis. 2009 Mar 1; 199(5): 625-32. doi: 10.1086/596743.
66. Verkaik N.J. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia / N.J. Verkaik, H.A. Boelens, C.P. de Vogel et al. // Eur. J. Clin. Microbiol. Infect. Dis. 2010 May; 29(5): 509-18. doi: 10.1007/s10096-010-0888-0.
67. Verkaik N.J., van Wamel W.J., van Belkum A. Immunothe–rapeutic approaches against Staphylococcus aureus // Immunotherapy. 2011 Sep; 3(9): 1063-73. doi: 10.2217/imt.11.84.
68. Wertheim H.F. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers / H.F. Wertheim, M.C. Vos, A. Ott et al. // Lancet. 2004 Aug 21–27; 364(9435): 703-5. doi: 10.1016/S0140-6736(04)16897-9.
69. Zielinski C.E. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β / C.E. Zielinski, F. Mele, D. Aschenbrenner et al. // Nature. 2012 Apr 26; 484(7395): 514-8. doi: 10.1038/nature10957.
70. Zouali M., Richard Y. Marginal zone B-cells, a gatekeeper of innate immunity // Front Immunol. 2011 Dec 13; 2: 63. doi: 10.3389/fimmu.2011.00063.

/171-1.jpg)
/175-1.jpg)
/172-1.jpg)
/176-1.jpg)
/177-1.jpg)
/178-1.jpg)